ER-phagy, VAMP7-Dependent Secretion of RTN3 and Neurite Growth
Authors
José Wojnacki, Sébastien Nola, Philippe Bun, Béatrice Cholley, Francesca Filippini, Mary T. Pressé, Joanna Lipecka, Sin Man Lam, Julie N’guyen, Axelle Simon, Amine Ouslimani, Guanghou Shui, Claudio Marcelo Fader, Maria Isabel Colombo, Ida Chiara Guerrera and Thierry Galli. Contacts: Thierry Galli thierry.galli@inserm.fr
Link to the original articleYear of publication
2020
Journal
Cell Reports
Abstract
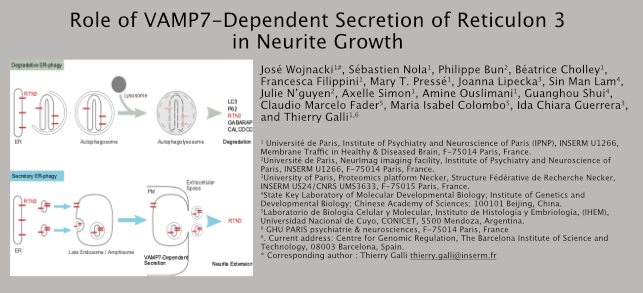
Here we found that axonal growth is affected by partial starvation and drugs which activate (Rapamycin, Resveratrol, Torine-1) or inhibit (Spautin-1) autophagy. Partial starvation, achieved by diluting aminoacids, vitamins and N2 supplement of the neuronal culture medium, stimulates axonal growth. Autophagy activators stimulate axonal growth, without affecting axonal polarization. Paradoxically, Spautin-1 also stimulates axonal growth but in a radically different manner since it also inhibits axonal polarization with treated neurons showing multiple axons instead of only one. We then took advantage of PC12 cells, a model of neuronal-like cells which can be easily genetically manipulated. We characterized VAMP7- and ATG5-KO cells. VAMP7, a secretory late endosome vesicular SNARE, was indeed previously shown to mediate NGF-evoked neurite growth in PC12 cells. VAMP7 had previously been involved in autophagy, particularly in the fly where VAMP8 is not present. ATG5 is an essential early component of macroautophagy. We confirmed that VAMP7 KO cells show decreased whereas ATG5 KO cells show increased neurite growth. Rapamycin only increases growth of the longest neurite and this effect is not seen in VAMP7 KO cells. Thorough lipidomics and proteomics and cell content and their secretome, validation of hits by western blotting, and exploration of the effect of cellular expression of an anti-VAMP7 synthetic antibody and the Longin amino-terminal domain of VAMP7 then lead us to the concept that ER elements destined to be degraded by ER-phagy are also released in large extracellular vesicles. This secretory route, which we refer to as secretory ER-phagy (SERP), allows for the release of ER-phagy-related molecules reticulons 1, 3 and 4, atlastins 1 and 3, Calcoco 1, and LC3-II. SERP is greatly enhanced when degradative ER-phagy is blocked such as by knocking out ATG5 or impairment of lysosomal pH by V-ATPase inhibitor BafilomycineA1. SERP is strongly inhibited by knocking out VAMP7. Expression of a VAMP7 synthetic antibody prevents starvation-induced axonal growth and expression of the Longin domain strongly affects the subcellular localization of reticulon 3 upon Rapamycin. In conclusion, we propose that VAMP7-dependent SERP is a crucial mechanism in neurite growth.
Graphical abstract
Role of VAMP7-Dependent Secretion of Reticulon 3 in Neurite Growth