BAG6, a new receptor for mitophagy?
Authors
Romain Ragimbeau, Leila El Kebriti, Salwa Sebti, Elise Fourgous, Abdelhay Boulahtouf, Giuseppe Arena, Lucile Espert, Andrei Turtoi, Céline Gongora, Nadine Houédé, Sophie Pattingre. Contact: sophie.pattingre@inserm.fr
Link to the original articleYear of publication
2021
Journal
FASEB J.
Abstract
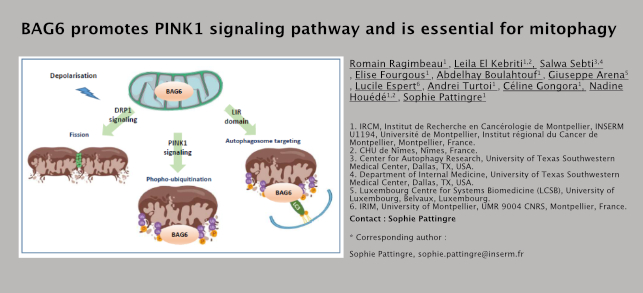
BAG6: a new receptor for mitophagy? The co-chaperone Bcl-2-associated athanogen-6 (BAG6) is a nucleocytoplasmic shuttling protein involved in protein quality control. Our previous work demonstrated that BAG6 is essential for autophagy by regulating the intracellular localization of the acetyltransferase EP300, and thus, modifying the accessibility to its substrates (TP53 in the nucleus and autophagy-related proteins in the cytoplasm). In this paper, we further investigated the role of BAG6 localization in the cytoplasm. We then demonstrated that BAG6 is localized in the mitochondria in all the cell lines tested (HEK293, MEFs, SW480 and in transfected BAG6 LoVo null cells). Specifically, BAG6 is expressed in the mitochondrial matrix under basal conditions, and translocates to the outer mitochondrial membrane after mitochondrial depolarization with carbonyl cyanide m-chlorophenyl hydrazine, a mitochondrial uncoupler that induces mitophagy. We wondered about BAG6 function in mitophagy and showed that BAG6 is positively involved in all the steps of the process: mitochondrial fission, PINK1/PARKIN signaling, and the specific targeting of the mitochondria into the autophagosome via a LIR domain. In SW480 cells, the deletion of BAG6 expression by shRNA abrogates its ability to induce mitophagy and PINK1 accumulation. On the reverse, its ectopic expression in LoVo colon cancer cells, which do not express endogenous BAG6, reduces the size of the mitochondria, induces mitophagy, leads to the activation of the PINK1/PARKIN pathway and to the phospho-ubiquitination of mitochondrial proteins. Finally, we noticed that BAG6 contains two LIR (LC3-interacting Region) domains specifically found in receptors for selective autophagy and responsible for the interaction with LC3 and for autophagosome selectivity. Site-directed mutagenesis showed that the C-terminal LIR domain of BAG6 is essential for its ability to stimulate mitophagy and its interaction with LC3. In conclusion, we propose that BAG6 is a novel mitophagy receptor or adaptor that induces mitochondrial fission, PINK1/PARKIN signaling and mitophagy in a LIR-dependent manner. BAG6 could act as a molecular platform able to interact with different actors of the mitophagic machinery.
Graphical abstract
BAG6 promotes PINK1 signaling pathway and is essential for mitophagy