The SEACIT complex is involved in the maintenance of vacuole/mitochondria contact sites and controls mitophagy
Authors
Yinxing Ma1 , Alexis Moors1 , Nadine Camougrand2, 3, 4 and Svetlana Dokudovskaya1, 4*
Link to the original articleYear of publication
2019
Journal
Cell Mol Life Sci.
Affiliation
1 CNRS UMR 8126, Université Paris-Sud 11, Institut Gustave Roussy, 114, rue Edouard Vaillant, 94805, Villejuif, France 2 CNRS, IBGC, UMR 5095, 1, rue Camille Saint-Saens, F-33000 Bordeaux, France 3 Université de Bordeaux, IBGC, 1, rue Camille Saint-Saens, F-33000 Bordeaux, France 4 These authors contributed equally to the work
Abstract
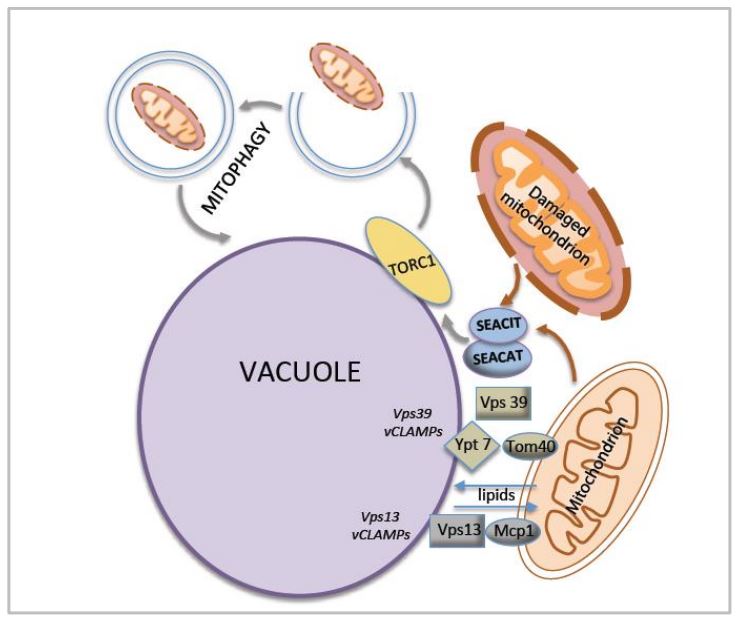
The major signaling pathway that regulates cell growth and metabolism is under the control of the target of rapamycin complex 1 (TORC1). In yeast S. cerevisiae the SEA complex, composed of SEACIT and SEACAT subcomplexes is one of the TORC1 upstream regulators involved in the response to amino acid availability and autophagy modulation. We performed analysis of the expression, interactions and localization of SEA complex proteins under different conditions, varying parameters such as sugar source, nitrogen availability and growth phase. Our results show that the SEA complex promotes mitochondria degradation either by mitophagy or by general autophagy. In addition, the SEACIT subcomplex is involved in the maintenance of the vacuole–mitochondria contact sites (vCLAMPs). Before our study, vCLAMPs were thought to be involved mainly in lipid exchange. Our results suggest a possible novel function for vCLAMPs—sensing the integrity and functionality of mitochondria. Given that the degradation of damaged mitochondria takes place in the vacuole via autophagy and/or mitophagy, and that the key players of this process—TORC1 and the SEA complex—reside at the vacuole membrane, it would be “spatially beneficial” for the cells to attribute this function (at least partially) to vCLAMPs. When detected by a vCLAMP, information about a dysfunctional organelle can be immediately transferred to the adjacent TORC1 network in order to initiate degradation. It is not impossible that the SEA complex might share the “sensing” activity with vCLAMPs or can have a potential regulatory function in this process, given that the SEA complex interacts with mitochondria membrane proteins and maintains vCLAMPs.
Graphical abstract