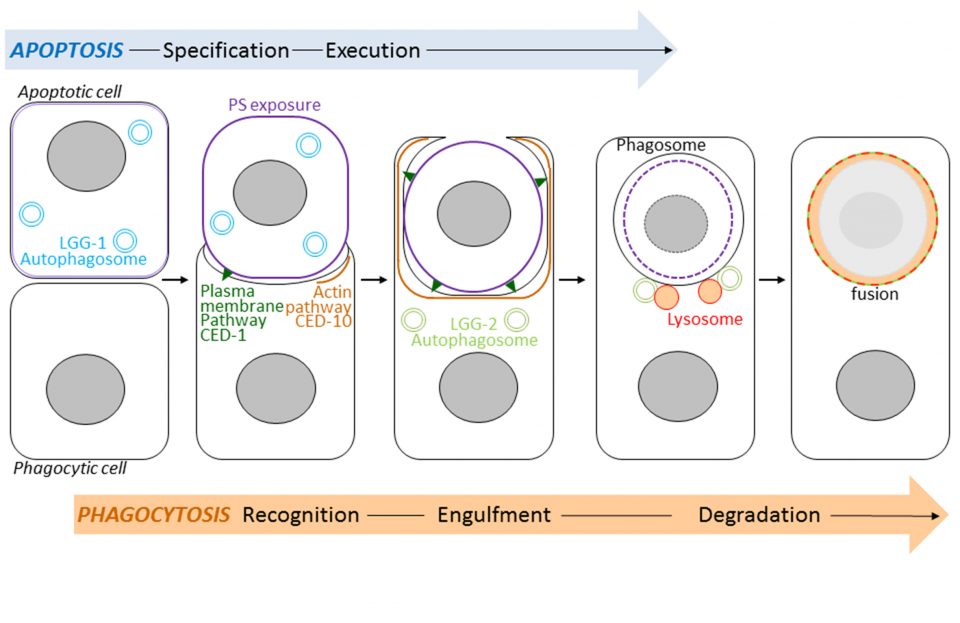
The autophagic proteins GABARAP/LGG-1 and LC3/LGG-2 possess specific functions in the exposure of phosphatidylserine and the degradation of apoptotic cells.
Authors
Céline Jenzer,Elena Simionato,Céline Largeau,Vincent Scarcelli,Christophe Lefebvre & Renaud Legouis
Link to the original articleYear of publication
2018
Journal
Autophagy
Abstract
Autophagy, which means self-eating, is a mechanism for degrading and recycling of cellular constituents. Double membrane vesicles called autophagosomes sequester cytoplasmic material and then fuse with lysosomes that digest their contents. This process is involved in development, longevity and cell death. Autophagy interacts strongly with a cell death program called apoptosis. Following its apoptosis, the cell is phagocytosed by the phagocytic cells forming a phagosome which then fuse with lysosomes causing its degradation (Figure). This process is called phagocytosis of apoptotic bodies. Using the model animal Caenorhabditis elegans, Renaud Legouis’s laboratory has identified the existence of new functions of autophagy that participate in the phagocytosis of apoptotic bodies. Using genetic and cellular approaches, such as light and electron microscopy, the authors have shown that the autophagic proteins LGG-1 and LGG-2, homologs of the human proteins GABARAP and LC3, play specific roles in the degradation of apoptotic cells. The LGG-1 protein is involved in the exposure at the surface of apoptotic cell of the lipid phosphatidylserine, serving as a death signal and causing its recognition by the phagocytic cell. The LGG-2 protein is specifically required later in the process to allow the maturation and degradation of the phagosome. The results of this study show the existence of several levels of interactions between autophagy and apoptosis. Understanding these relationships is important at the medical level because manipulating autophagy is a therapeutic issue under study in various human pathologies such as cancers or neurodegenerative diseases.
Graphical abstract