Implication of a lysosomal antigen in the pathogenesis of lupus
Authors
Wilhelm, M., Bonam, S.R., Schall, N., Bendorius, M., Korganow, A.-A., Lumbroso C., Muller, S. J Autoimmun. 2021 Jun;120:102633. Pubmed PMID: 33932829 Contact: Prof. Sylviane Muller, CNRS UMR7242 Biotechnologie et signalisation cellulaire, Université de Strasbourg/ Institut du Médicament de Strasbourg (IMS); Institut de science et d’ingénierie supramoléculaire 67000 Strasbourg; sylviane.muller@unistra.fr ORCID 0000-0002-0481-0620
Link to the original articleAbstract
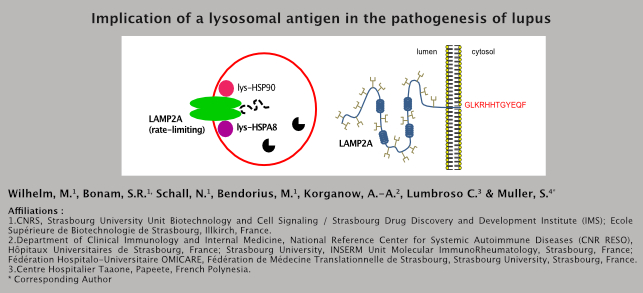
Autoantibodies to components of the autophagy process in SLE Naturally-occurring autoantibodies to certain components of autophagy processes have been described in a few autoimmune diseases, but their fine specificity, their relationships with clinical phenotypes, and their potential pathogenic functions remain elusive. We have explored IgG autoantibodies reacting with a panel of cytoplasmic endosomal/lysosomal antigens and individual heat-shock proteins (HSP), all of which share links to autophagy. The choice of these potential autoantigens was dictated by the fact that autophagy has been found to be deregulated in a number of pathologies, including autoimmune diseases, and notably systemic lupus erythematosus (SLE). Investigations were conducted in the context of patients with SLE, Sjögren’s syndrome, rheumatoid arthritis, and systemic sclerosis (total 185 patients from France, Italy and French Polynesia), and in parallel, in MRL/lpr and (NZBxNZW)F1 lupus-prone mice, and non-obese diabetic mice. This screening study showed that sera from autoimmune patients and from MRL/lpr and NZB/W mice reacted with the C-terminal residues of lysosome-associated membrane glycoprotein (LAMP)2A, a receptor for cytosolic proteins targeted for degradation via chaperone-mediated autophagy. No cross-reaction was observed with LAMP2B or LAMP2C variants, with dsDNA or mononucleosomes, or with HSPA8. In lupus patients, there was a strong association of the level of serum circulating anti-LAMP2A antibodies and lupus flares (64.7%), anti-phospholipid syndrome (76.5%), dermatological features (52.6%), joint inflammation (52.6%), and renal failure (33.3%), as well as with the SLEDAI-2K score of severity (r=0.3389; P=0.0324). Moreover, administering chromatography-purified LAMP2A autoantibodies to MRL/lpr mice accelerated mortality. Furthermore, flow cytometry revealed unexpected elevated cell-surface expression of LAMP2A on MRL/lpr B cells. These findings reveal the involvement of a new class of autoantibodies targeting the C-terminus of LAMP2A. These autoantibodies could affect the autophagy process, which is abnormally upregulated in lupus. The data presented support a novel connection between autophagy dysregulation, autoimmune processes and pathophysiology in lupus.
Graphical abstract