The AMPK-Sirtuin 1-YAP axis is regulated by fluid flow intensity and controls autophagy flux in kidney epithelial cells
Authors
Aurore Claude-Taupin, Pierre Isnard, Alessia Bagattin, Nicolas Kuperwasser, Federica Roccio, Biagina Ruscica, Nicolas Goudin, Meriem Garfa-Traoré, Alice Regnier, Lisa Turinsky, Martine Burtin, Marc Foretz, Marco Pontoglio, Etienne Morel, Benoit Viollet, Fabiola Terzi, Patrice Codogno & Nicolas Dupont
Link to the original articleYear of publication
2023
Journal
Nature Communications
Affiliation
Institut Necker Enfants Malades
Abstract
Shear stress generated by urinary fluid flow is an important regulator of renal function. Its dysregulation is observed in various chronic and acute kidney diseases. Previously, we demonstrated that primary cilium-dependent autophagy allows kidney epithelial cells to adapt their metabolism in response to fluid flow. Here, we show that nuclear YAP/TAZ negatively regulates autophagy flux in kidney epithelial cells subjected to fluid flow. This crosstalk is supported by a primary cilium-dependent activation of AMPK and SIRT1, independently of the Hippo pathway. We confirm the relevance of the YAP/TAZ-autophagy molecular dialog in vivo using a zebrafish model of kidney development and a unilateral ureteral obstruction mouse model. In addition, an in vitro assay simulating pathological accelerated flow observed at early stages of chronic kidney disease (CKD) activates YAP, leading to a primary cilium-dependent inhibition of autophagic flux. We confirm this YAP/autophagy relationship in renal biopsies from patients suffering from diabetic kidney disease (DKD), the leading cause of CKD. Our findings demonstrate the importance of YAP/TAZ and autophagy in the translation of fluid flow into cellular and physiological responses. Dysregulation of this pathway is associated with the early onset of CKD.
Graphical abstract
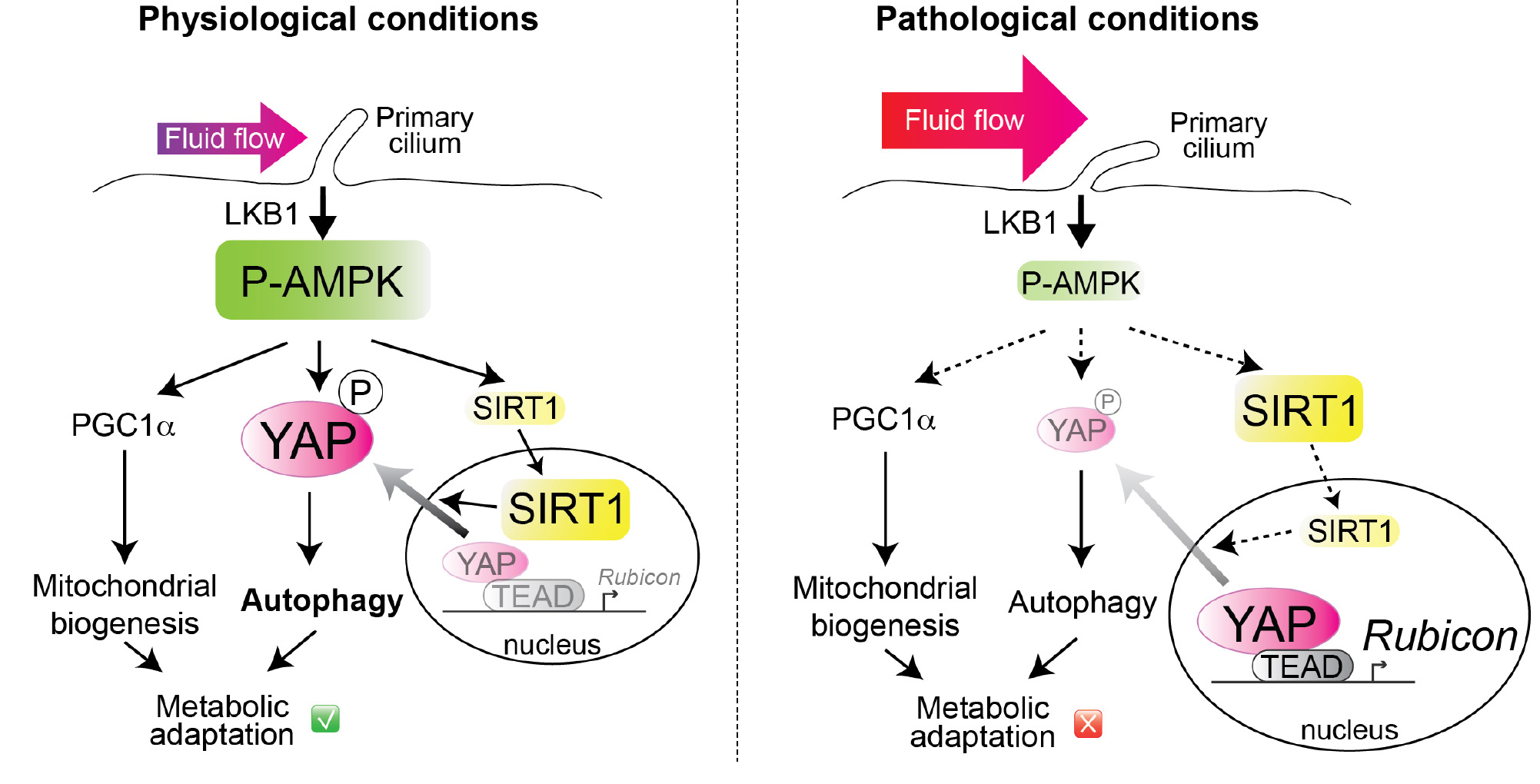
In physiological conditions, the primary cilium-dependent activation of AMPK ensures the stimulation of 3 pathways: (i) SIRT1 activation is necessary to induce YAP exit from the nucleus (ii) AMPK-dependent phosphorylation of YAP on S61 sequestrate YAP in the cytosol, thus inhibiting the transcription of Rubicon, an autophagy suppressor. (iii) Increase of mitochondrial biogenesis. This metabolic reprogramming supports energy- consuming cellular processes such as glucose reabsorption and gluconeogenesis. In pathological conditions, the primary cilium-dependent activation of AMPK is impaired, resulting notably in the accumulation of nuclear YAP and inhibition of autophagy.