Exosomes and unconventional secretion
Université MontpellierCampus Triolet, cc107
Montpellier 34095 - Montpellier
Site web - michel.vidal@umontpellier.fr -
Principal investigator
Michel Vidal
Research themes
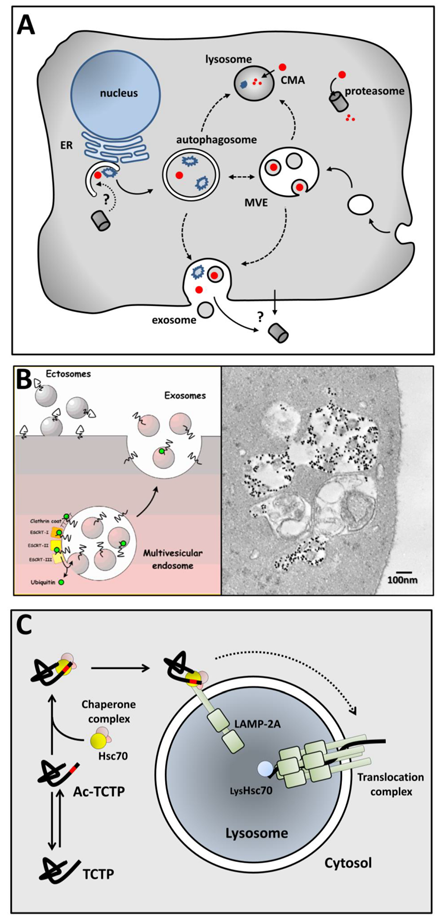
Maintaining cellular proteostasis is accomplished by two processes, biosynthesis vs removal of proteins, with the help of chaperone proteins. Removal of cytosolic proteins is carried out by different ways: proteasomal degradation, autophagic lysosomal degradation and unconventional secretion (Fig. A).
Cells use various mechanisms for unconventional secretion. Exosome secretion is one of these processes. Exosomes are membrane vesicles (60-100nm diameter) formed by inward budding of the endosomal membrane and thus trapping cytosol inside. The so-formed multivesicular bodies then fuse with the plasma membrane releasing intraluminal vesicles, at this point called exosomes, in the extracellular medium. This exosomal secretion pathway is essential for the last step of erythroid differentiation, allowing for removal of useless proteins in erythrocyte (Fig. B). Other mechanisms of unconventional secretion involve either direct transport of cytosolic proteins across membranes or fusion of autophagosome with the plasma membrane.
We are interested by factors determining the pathways through which proteins are specifically removed. One of our objectives is to understand the mechanisms involved in cellular TCTP (translationally controlled tumor protein) homeostasis. TCTP is a cytosolic multifunctional protein for which high cellular levels are generally poor prognostic for cancers. We had shown that TCTP is exosomally secreted by cells consecutively to p53 activation by irradiation. We have recently shown that TCTP is degraded by CMA (chaperone-mediated autophagy) when acetylated on a specific lysine (19KIREI) granting it with the pseudo-glutamine status essential in the KFERQ-like motif recognized by Hsc70 (Fig. C).
It is to note that TCTP is also secreted as a free protein (HRF: histamine-releasing factor) inducing histamine release from basophils. TCTP is secreted by an alternative pathway whose molecular mechanism is still not known. Preliminary experiments using ATG5 depleted MEFs suggest that secretory autophagy could be involved.
We are also interested by connections (regulation, junction, compensation) existing between these different removal pathways. For example, ATG5 depletion in MEFs leads to changes in exosomal protein content. We have also evidenced the presence of proteasome (20S) in our purified exosomal fractions and we are currently studying the mechanism of this proteasome release.
Descriptive figure
Publications
A. Bonhoure, A. Vallentin, M. Martin, A. Senff-Ribeiro, R. Amson, A. Telerman and M. Vidal (2017) Acetylation of Translationally Controlled Tumor Protein promotes its degradation through Chaperone-Mediated Autophagy. Eur. J. Cell Biol. DOI: 10.1016/j.ejcb.2016.12.002.
L. Blanc and M. Vidal (2017) New Insights into the function of Rab GTPases in the context of exosomal secretion. Small GTPases. DOI : 10.1080/21541248.2016.1264352.
L. Blanc and M. Vidal (2010) Reticulocyte membrane remodeling : contribution of the exosome pathway. Curr. Opin. Hematol. 17, 177-183.
A. Lespagnol, D. Duflaut, C. Beekman, L. Blanc, G. Fiucci, J.C. Marine, M. Vidal, R. Amson, A. Telerman. (2008) Exosome secretion, including the DNA damage-induced p53-dependent secretory pathway, is severely compromised in TSAP6/Steap3-null mice. Cell Death Differ. 15 (11), 1723-1733.
A. de Gassart, C. Géminard, D. Hoekstra and M. Vidal (2004) Exosome secretion: the art of reutilizing nonrecycled proteins? Traffic 5, 896-903.
Composition de l'équipe
Pascale Bette-Bobillo, MCU
Anne Bonhoure, adjointe technique
Michel Vidal, DR2