Herpesviruses and Autophagy
I2BC Institut de Biologie Intégrative de la CelluleDépartement de Virologie
Gif sur Yvette.
- Gif sur Yvette
Site web - -
Principal investigator
Pr Audrey Esclatine

Research themes
Our team, historically located in the Faculty of Pharmacy of Univ Paris-Sud, has been interested for many years now in viruses belonging to the Herpesviridae family that infect humans. During these last years, we have worked on Herpes Simplex virus type 1 (HSV-1), human cytomegalovirus (HCMV) and Epstein-Barr virus (EBV). These viruses are known for their ability to largely modulate and counteract antiviral cellular mechanisms, in particular because of the important size of their genome (for viruses, of course!) and their high coding capacity. We have thus studied relationships existing between viral infection and regulation of the autophagic process in different contexts.
Viral proteins specialized in autophagy modulation
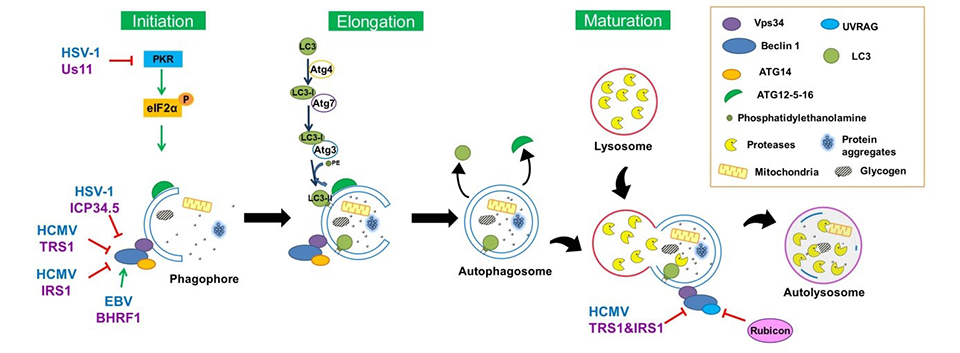
In our laboratory, we have identified several autophagy-modulating proteins from different Herpesviruses and we have characterized their mechanisms of action. Us11, a protein expressed by HSV-1, is able to block autophagosome formation, both in the course of infection and when ectopically expressed. Us11 acts by preventing the kinase activity of PKR, which can detect double stranded RNA coming from viruses leading to inhibition of protein synthesis and stimulation of autophagy (5). Us11 thus complements the defense arsenal of HSV-1 against autophagy, since another protein, ICP34.5, had been initially identified by Beth Levine’s laboratory in 2002. Us11 acts later than ICP34.5 in the productive growth cycle of HSV-1. We have also demonstrated that HCMV prevents autophagy thanks to at least two inhibitory proteins (3, 6, 7). The two highly homologous proteins TRS1 and IRS1, block the whole autophagic process by different mechanisms. Indeed, when expressed separately, they are able to decrease autophagosome formation by interaction with the coiled coil domain of Beclin1. When expressed simultaneously, they are also able to block the autophagic degradation, leading to an accumulation of LC3 (3). More recently, we have been able to demonstrate, in collaboration with Vincent Maréchal (CIMI, Hôpital Pitié Salpêtrière, Paris), that an EBV protein (BHRF1) interacts with Beclin 1 and can stimulate autophagosome formation.
Why should viruses modulate autophagy?
Numerous viruses, including several Herpesviruses, are able to modulate autophagy for different reasons (1, 2). Some viruses can block autophagy which sometimes acts as an antiviral defense mechanism established by the cell. But, viruses can also modulate autophagy in order to hijack all or parts of the autophagic process to optimize their productive growth cycle or to mitigate the impact of antiviral immunity.
We have demonstrated in collaboration with the Italian laboratory of Pr Maria-Teresa Sciortino that autophagy can be hijacked by HSV-1 to its own profit (4). Indeed, infection of monocytic cells by HSV-1 leads to a transient activation of autophagy at the early steps of infection in order to improve its entry by endocytosis in the cells. HCMV, although inhibiting the autophagic process, also hijacks parts of the autophagic machinery to improve its production in human fibroblasts. (3). Indeed, invalidation of different autophagic genes leads to a global decrease of the viral production, demonstrating the proviral effect of autophagy on HCMV. EBV also takes advantage of autophagy activation mediated by the BHRF1 protein to limit the cell-autonomous antiviral immune response, by modifying mitochondria morphology and fate.
These studies were funded by ANR and by Région Ile de France.
et par la Région Ile de France.
Descriptive figure
Publications
1. M. Lussignol et A. Esclatine (2017) Herpesvirus and Autophagy: « All Right, Everybody Be Cool, This Is a Robbery!, Viruses, vol. 9, nᵒ 12, déc. 2017.
2. I. Vergne, F. Lafont, L. Espert, A. Esclatine, et M. Biard-Piechaczyk (2017) Autophagie, protéines ATG et maladies infectieuses, médecine/sciences, vol. 33, nᵒ 3, p. 312-318
3. L. Mouna, E. Hernandez, D. Bonte, R. Brost, L. Amazit, L. R. Delgui, W. Brune, A. P. Geballe, I. Beau, et A. Esclatine (2016), « Analysis of the role of autophagy inhibition by two complementary human cytomegalovirus BECN1/Beclin 1-binding proteins, Autophagy, vol. 12, nᵒ 2, p. 327-342
4. G. Siracusano, A. Venuti, D. Lombardo, A. Mastino, A. Esclatine, et M. T. Sciortino, (2016) Early activation of MyD88-mediated autophagy sustains HSV-1 replication in human monocytic THP-1 cells, Scientific Reports, vol. 6, p. 31302
5. Lussignol M, Queval C, Bernet-Camard MF, Cotte-Laffitte J, Beau I, Codogno P, Esclatine A (2013) The Herpes Simplex Virus 1 Us11 Protein Inhibits Autophagy through Its Interaction with the Protein Kinase PKR. J Virol 87 : 859-871
6. Chaumorcel M, Lussignol M, Mouna L, Cavignac Y, Fahie K, Cotte-Laffitte J, Geballe A, Brune W, Beau I, Codogno P, Esclatine A (2012) The human cytomegalovirus protein TRS1 inhibits autophagy via its interaction with Beclin 1. J Virol 86 : 2571-2584
7. Chaumorcel M, Souquère S, Pierron G, Codogno P, Esclatine A. (2008) Human cytomegalovirus controls a new autophagy-dependent cellular antiviral defense mechanism. Autophagy 4(1):46-53
Composition de l'équipe
Audrey Esclatine, Professeur de Virologie, Univ Paris-Sud
Marion Lussignol, Maître de Conférences, Univ Paris-Sud
Eva Hernandez-Djebali, Ingénieure d’étude, Univ Paris-Sud
Clémence Taisne, Doctorante, Univ Paris-Sud
Damien Glon, Etudiant en Master 2, Univ Paris-Sud