Regulation of CD4 T cell differentiation in cancer
« Lipides, Nutrition, Cancer »Inserm U1231, Université de Bourgogne, Agrosup Dijon
UFR des Sciences de Santé, 7 Bd Jeanne d’Arc, 21000 Dijon
- Dijon
Site web - lionel.apetoh@inserm.fr - 0380393443
Principal investigator
Lionel Apetoh

Research themes
Our team studies CD4 T cell properties and role in anti-tumor immune responses. While these cells do not necessary display direct anti-tumor effects, they orchestrate the cooperation between immune cells required to eliminate cancer cells. We aim at understanding the signaling pathways that control CD4 T cell differentiation and anti-tumor properties to develop new immunotherapeutic strategies.
Autophagy proteins have been shown to play important roles in innate immunity and antigen presentation. However, their cell-intrinsic functions in CD4 T cells has received less attention (Jacquin & Apetoh, Front. Immunol., 2018). We focus on a novel role for autophagy in modulating CD4 T cell differentiation and anti-tumor properties.
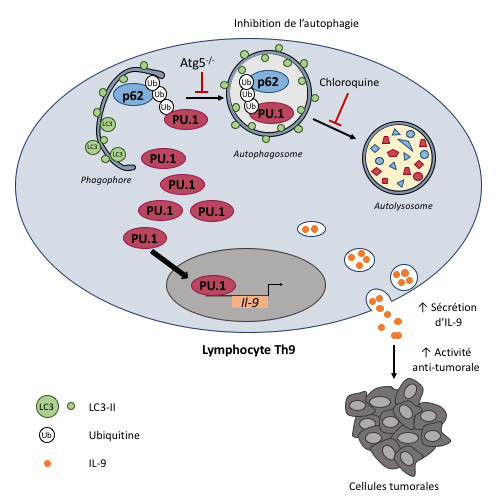
In our recent study, we showed that autophagy restrains the production of IL-9 by CD4 T cells differentiated into Th9 cells, a subset of effector cells with strong anti-tumor properties. Exploration of the underlying molecular mechanisms revealed that ubiquitination of PU.1, the master transcription factor of Th9 cells, leads to its specific recruitment by the autophagy receptor p62 and its subsequent degradation in autophagosomes. Thus, p62-dependent selective autophagy restrains the differentiation of Th9 cells without affecting the differentiation of CD4 T cells into other effector cell subsets such as Th1, Th2 and Th17. Moreover, pharmacological and genetic inhibition of autophagy lead to an increased anti-tumor activity of Th9 cells in vivo. This new role for autophagy in CD4 T cell differentiation that we aim to further characterize supports the importance of this process in anti-tumor immune responses mediated by CD4 T cells (Rivera Vargas et al., Nat. Commun., 2017 ; Isis Benoit-Lizon et al, Cell cycle, 2018).
Descriptive figure
Publications
Jacquin E and Apetoh L. Cell-Intrinsic Roles for Autophagy in Modulating CD4 T Cell Functions. Front. Immunol. 2018;9:1023.
Benoit-Lizon I, Jacquin E, Apetoh L. Selective autophagy restricts IL-9 secretion from T(H)9 cells: relevance in cancer growth. Cell Cycle. 2018;17(4):391-392.
Rivera Vargas T, Cai Z, Shen Y, Dosset M, Benoit-Lizon I, Martin T, Roussey A, Flavell RA, Ghiringhelli F, Apetoh L. Selective degradation of PU.1 during autophagy represses the differentiation and antitumour activity of T(H)9 cells. Nat Commun. 2017 Sep 15;8(1):559.
Rivera Vargas T, Benoit-Lizon I, Apetoh L. Rationale for stimulator of interferon genes-targeted cancer immunotherapy. Eur J Cancer. 2017 Apr;75:86-97.
Rivera Vargas T, Humblin E, Végran F, Ghiringhelli F, Apetoh L. T(H)9 cells in anti-tumor immunity. Semin Immunopathol. 2017 Jan;39(1):39-46.
Végran F, Berger H, Boidot R, Mignot G, Bruchard M, Dosset M, Chalmin F, Rébé C, Dérangère V, Ryffel B, Kato M, Prévost-Blondel A, Ghiringhelli F, Apetoh L. The transcription factor IRF1 dictates the IL-21 dependent anticancer functions of TH9 cells. Nat Immunol. 2014 Aug;15(8):758-66.
Composition de l'équipe
Alvaro Baeza Garcia, Postdoc
Isis Benoit-Lizon, PhD student
Ludivine Dal Zuffo, Research assistant
Alexandre Dremeau, Undergraduate student
Carole Fournier, Postdoc
Elise Jacquin, Postdoc
Zhiqiang Jiang, Postdoc
François Martin, Professor emeritus
Tiffany Martin, Research assistant
Andréa Mélis, Research assistant
Aurélie Roussey, Research assistant
Athina Stavridou, M2 student