Spatial Signalling
Tumour Biology, Barts Cancer InstituteQueen Mary University of London
John Vane Science Centre
Charterhouse Square
London EC1M 6BQ - London
Site web - -
Principal investigator
Dr Stéphanie Kermorgant

Research themes
Our research focuses on understanding the mechanisms of a molecule called c-Met in promoting cancer metastasis.
c-Met is overexpressed or mutated in a large number of tumours and has emerged as a major target for cancer therapy. c-Met is a tyrosine kinase receptor (RTK). Together with the protein that binds to it: its ligand, Hepatocyte Growth Factor (HGF), they are key players in tumour metastasis. They affect cell adhesion, migration, invasion, metalloproteinase activation and angiogenesis. Drugs against c-Met are being designed and some are being tested in patients in clinical trials.
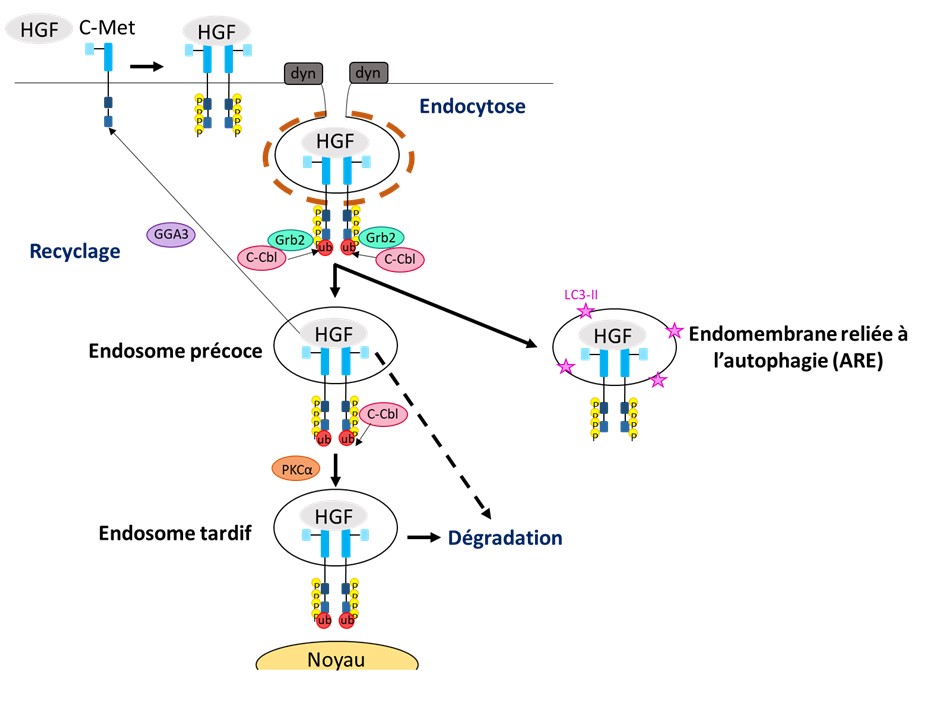
Receptors such as c-Met are normally present at the surface of transformed cells in the tumour mass, where they transmit information (signals) from the outside to the inside of the cell in order to change its behaviour (divide/don’t divide; move/don’t move). However, receptors can enter cells (endocytosis) and recently have been found to transmit signals from within the cell. This is a new scientific concept that changes our understanding of how receptors function.
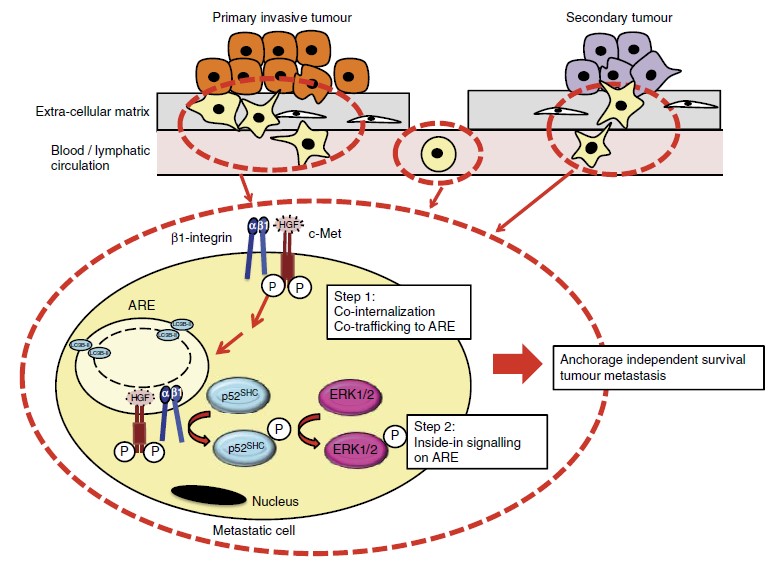
We study the signalling of c-Met in relation to its endosomal trafficking (how it is moved around the cell in vesicles) and the effects on tumour cell migration and invasion in vitro and in vivo. We use confocal microscopy, live imaging, biochemistry, trafficking / internalisation assays, functional assays, and in vivo tumorigenesis assays. We investigate the clinical relevance of our findings on patient samples through collaboration with clinicians. We work on breast, pancreatic, lung and ovarian cancer. We have reported that c-Met mutants found in cancer patients are oncogenic not only because they are highly activated but also because they signal on endosomes. We have shown that c-Met transmit distinct signalling pathways from different endosomes including a novel endosome we recently discovered, the “Autophagy Related Endomembrane”.
We anticipate that a better understanding of the molecular biology of intracellular c-Met will lead to improved cancer treatment.
Descriptive figure
Publications
– Barrow-McGee et al, Beta 1-integrin- c-Met cooperation reveals an inside-in survival signalling on Autophagy Related Endomembranes. Nature Communications (2016) 7:11942.
– Menard et al, Receptor Tyrosine Kinase c-Met controls the cytoskeleton from different endosomes via different pathways. Nature Communications (2014) 5:3907.
– Mai et al, Distinct c-Met Activation Mechanisms Induce Cell Rounding or Invasion Through Pathways Involving Integrins, RhoA, and Hip1. J Cell Sci (2014) 127:1938-52.
– Joffre et al, A direct role for Met endocytosis in tumorigenesis. Nat Cell Biol (2011) 13(7):827-37.
Composition de l'équipe
Bakhouche Bakhouche (post-doctorant)
Waleed Badreldin (oncologue, doctorant)
Brynna Hoggard (doctorante)
Alejandro Noval (doctorant)
Marie Nollet (post-doctorante)
Georgina Wood (oncologue, doctorante)