Cell Death and Autophagy in Atherosclerosis
Inserm UMR 1048/ Institut des Maladies Métaboliques et Cardiovasculaires, Université Paul Sabatier, Toulouse 3.Equipe « Lipides, Peroxydation, Signalisation dans les maladies vasculaires »
1 avenue Jean Poulhès
31432 Toulouse, France - Toulouse
Site web - cecile.vindis@inserm.fr -
Principal investigator
Cécile VINDIS, DR2 INSERM

Research themes
Our aim is to discover new mechanisms involved in atherosclerotic plaque rupture in order to identify targets that contribute to plaque fragilization and instability. Initially, our research activity was focused on apoptotic signaling pathways and cellular mechanisms induced by atherogenic oxidized lipids. Indeed, apoptosis can affect all the vascular cell types and is strongly related to atherosclerotic lesion stage and plaque rupture. Our previous work has demonstrated the major role of calcium signaling and the mitochondria in the regulation of vascular cell death. Recently we started to investigate the adaptive and cell survival pathways activated in parallel with apoptotic pathways induced by atherogenic lipids. We have shown the induction of ER stress and autophagy which takes part through beclin-1 to the phosphatidylserine exposure process as “eat-me” signal required for dead-cell clearance. Now, our works are focused on the role of a selective autophagy process named mitophagy. We aim to delineate the role and the regulation of mitophagy as a protective mechanism against vascular smooth muscle cell death-mediated atherosclerotic plaque fragilization and vulnerability. We hypothesized that the engulfment of defective mitochondria by autophagosomes could limit the release of proapoptotic proteins into the cytosol and safeguards plaque cells. Our results show that atherogenic lipid factors trigger the mitophagy machinery in human vascular smooth muscle cell. Using loss- or gain-of-expression approaches we reported that mitophagy through Parkin and PINK1 had critical consequences on cell fate by enhancing apoptosis or by favouring cell survival. The next step is to assess the occurrence and the role of mitophagy in animal models of atherosclerosis invalidated for mitophagy proteins. Ultimately we hope to raise the interest to target selectively mitophagy as a new strategy to stabilize atherosclerotic plaque.
Descriptive figure
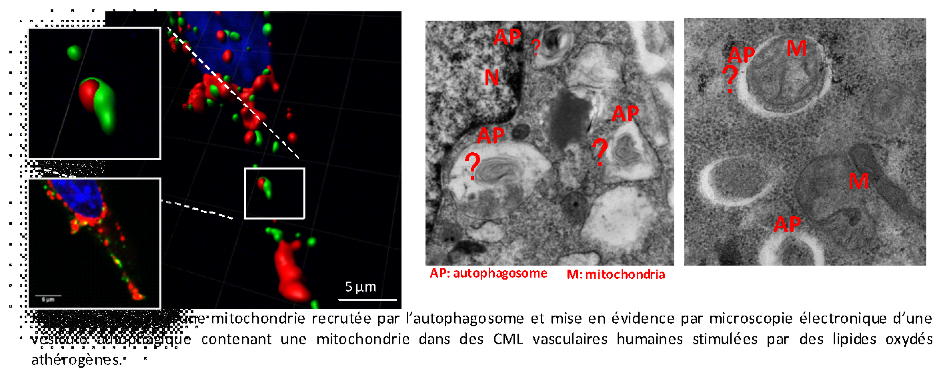
Publications
– Vindis C. Autophagy: an emerging therapeutic target in vascular diseases. Br J Pharmacol. 2015 May;172(9):2167-78.
– Laurent AC, Bisserier M, Lucas A, Tortosa F, Roumieux M, De Régibus A, Swiader A, Sainte-Marie Y, Heymes C, Vindis C, Lezoualc’h F. Exchange protein directly activated by cAMP 1 promotes autophagy during cardiomyocyte hypertrophy. Cardiovasc Res. 2015 Jan 1;105(1):55-64.
– Larroque-Cardoso P, Swiader A, Ingueneau C, Nègre-Salvayre A, Elbaz M, Reyland ME, Salvayre R, Vindis C. Role of protein kinase C δ in ER stress and apoptosis induced by oxidized LDL in human vascular smooth muscle cells. Cell Death Dis. 2013 Feb 28;4:e520.
– Muller C, Salvayre R, Nègre-Salvayre A, Vindis C. Oxidized LDLs trigger endoplasmic reticulum stress and autophagy: prevention by HDLs. Autophagy. 2011 May;7(5):541-3.
– Muller C, Salvayre R, Nègre-Salvayre A, Vindis C. HDLs inhibit endoplasmic reticulum stress and autophagic response induced by oxidized LDLs. Cell Death Differ. 2011 May;18(5):817-28.
Composition de l'équipe
Meyer ELBAZ, PU-PH
Hripsime NAHAPETYAN, doctorante
Jonathan BONNEVILLE, doctorant
Nicolas Montée, PH
Cécile INGUENEAU, MCU-PH
Audrey SWIADER, TR
Julien FACCINI, AI