Host-Virus interactions
Institut Cochin, Inserm U1016-CNRS UMR8104Université Paris Descartes
27, rue du faubourg Saint Jacques
75014 Paris - Paris
Site web - clarisse.berlioz@inserm.fr - 0140516575
Principal investigator
Clarisse Berlioz-Torrent et Stéphane Emiliani

Research themes
The Human Immunodeficiency Virus (HIV) infection is characterized by high levels of viral replication and spread even during the clinically latent stage of AIDS. Because of its limited coding capacity, HIV heavily relies on the host cellular machinery to replicate. The objective of “Host-virus interactions” team at the Institut Cochin (Paris) is to explore the relationship between HIV-1 and the host cell in order to gain insights into the molecular mechanisms underlying retroviral pathogenesis. Over the past 7 years, Clarisse Berlioz-Torrent’s group elucidated the role of trafficking and degradative pathways during HIV release, and decipher the cellular and anti-viral functions on HIV release of the restriction factor BST2 (bone marrow stromal antigen 2)/Tetherin.
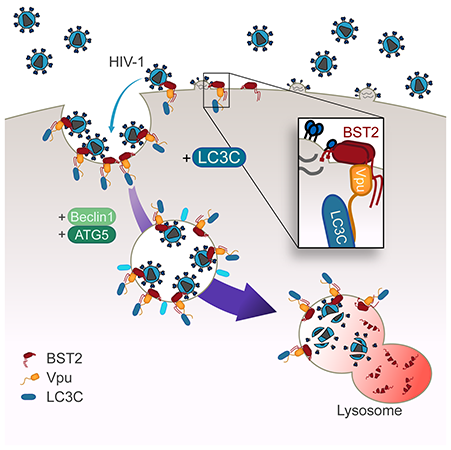
BST2 is a restriction factor that considerably reduces enveloped viruses release by physically trapping de novo formed mature viral particles at the surface of infected cells. The antiviral activity of BST2 relies on its presence at the viral budding site and on its ability to be incorporated into nascent budding virions, bridging virions and cellular membranes). Lentiviruses have adopted diverse strategies to antagonize this restriction and further optimize viral release, notably by encoding viral proteins, such as HIV-1 Vpu protein, able to interact with BST2, down-regulate cell surface expression of BST2 and displace it from viral budding sites
Clarisse Berlioz-Torrent’s group recently revealed new role of autophagic proteins in HIV countermeasure against BST2 restriction. By exploring the molecular mechanism controlling this viral restriction, this team discovered that some components of autophagic pathway, the ATG5, Beclin 1 and LC3C autophagic proteins, are required for HIV-1 to relieve this restriction. Autophagy is a highly conserved degradative pathway that maintains cellular homeostasis, responds to pathological processes and combats infections. The team showed that knocking-down ATG5, Beclin 1 and LC3C expression, impaired the viral release and propagation: viruses accumulated at the cell surface and in intracellular compartments. The team deciphered the mechanism involved: Through its interaction with LC3C, HIV-1 Vpu protein favors the targeting of BST2 molecules present at the budding site to degradation via a non-canonical autophagy pathway. This work reveals that HIV-1 through its Vpu protein may use a non-canonical autophagy pathway, reminiscent to a LAP process, to counteract efficiently the viral restriction mediated by BST2/Tetherin factor on the viral release. This work was funded by ANRS and SIDACTION
Descriptive figure
Publications
Articles originaux sélectionnés
1 – Roy N., Pacini G., Berlioz-Torrent C., and Janvier K. Characterization of E3 ligases involved in lysosomal sorting of the HIV-1 restriction factor BST2. J Cell Sci. 2017 May 1; 130(9): 1596-1611.
2 – Madjo U.*, Leymarie, O.*, Fremont, F., Kuster, A., Gourhand, V., Gallois-Montbrun, S., Janvier, K. and C. Berlioz-Torrent. LC3C contributes to Vpu-mediated antagonism of BST2/Tetherin restriction on HIV-1 release through a non canonical autophagy pathway. Cell Rep. 2016 Nov 22;17(9):2221-2233. *Co-first authors.
3 – Fremont, S., Gerard, A., Galloux, M., Janvier, K., Karess, R. E., Berlioz-Torrent, C. Response to Luca L Fava and colleagues EMBO Rep 2015; 16(10):1237-8.
4 – Fremont, S., Gerard, A., Galloux, M., Janvier, K., Karess, R. E., Berlioz-Torrent, C. Beclin-1 is required for chromosome congression and proper outer kinetochore assembly EMBO Rep 2013; 14(4):364-72.
5 – Caillet M, Janvier K, Pelchen-Matthews A, Delcroix-Genête D, Camus G, Marsh M, Berlioz-Torrent C. Rab7A is required for efficient production of infectious HIV-1. PLoS Pathog 2011 Nov;7(11):e1002347.
6 – Janvier K§, Pelchen–Matthews A, Renaud J-B, Caillet M, Marsh M, Berlioz-Torrent C§. The ESCRT-0 component HRS is required for HIV-1 Vpu-mediated BST2/Tetherin down-regulation. PloS Pathog 2011 7(2): e1001265. §Corresponding authors.
7 – Estrabaud E, Le Rouzic E, Lopez-Vergès S, Morel M, Belaïdouni N, Benarous R, Transy C, Berlioz-Torrent C, Margottin-Goguet F. (2007). Regulated degradation of the HIV-1 Vpu protein through a betaTrCP-independent pathway limits the release of viral particles. PLoS Pathog. 2007 Jul 27;3(7):e104.
Revue
1 – Leymarie O, Lepont L, Berlioz-Torrent C. Canonical and Non-Canonical Autophagy in HIV-1 Replication Cycle.Viruses. 2017 Sep 23;9(10). pii: E270. doi: 10.3390/v9100270. Review.
2 – Roy, N. *, Pacini, G. *, Berlioz-Torrent, C., Janvier, K. Mechanisms underlying HIV-1 Vpu-mediated viral egress Front Microbiol 2014; 5:177. Review. *Co-first authors.
3 – Janvier, K.*, Roy, N., Berlioz-Torrent, C*. Role of the Endosomal ESCRT Machinery in HIV-1 Vpu-Induced Down- Regulation of BST2/Tetherin. Curr HIV Res 2012 10(4):315-20. Review. *Corresponding authors
Composition de l'équipe
Clarisse Berlioz-Torrent, DR2 INSERM *
Stéphane Emiliani, DR 2 CNRS
Marc Lavigne, CR Pasteur
Sarah Gallois Montbrun, CR2 INSERM
Katy Janvier CR1 CNRS*
Aurelia Kuster, Post-Doc*
Marianne El Barouk, Post-doc*
May Baikal, Post-doc
Sarah Hamoudi, Post-doc
Maria-José Lista, Post-doc
Sarah N’Da Konan, Post-doc
Sophie Goudey, PhD Student
Leslie Lepont, PhD Student*
Emmanuel Ségéral, Ingénieur d’étude
Margaux Versapuech, Assistant Ingénieur*
* Groupe de C. Berlioz-Torrent