Endothelial Physiopathology and Extracellular Vesicles
Hôpital Européen Georges Pompidou56 rue Leblanc - Paris
Site web - chantal.boulanger@inserm.fr - 33153988086
Principal investigator
Chantal M. Boulanger

Research themes
Our work is focused on the role of autophagy in endothelial cells and its involvement in the pathophysiology of diseases related to metabolic syndrome (combination of obesity, insulin resistance or type 2 diabetes, dyslipidemia and hypertension).
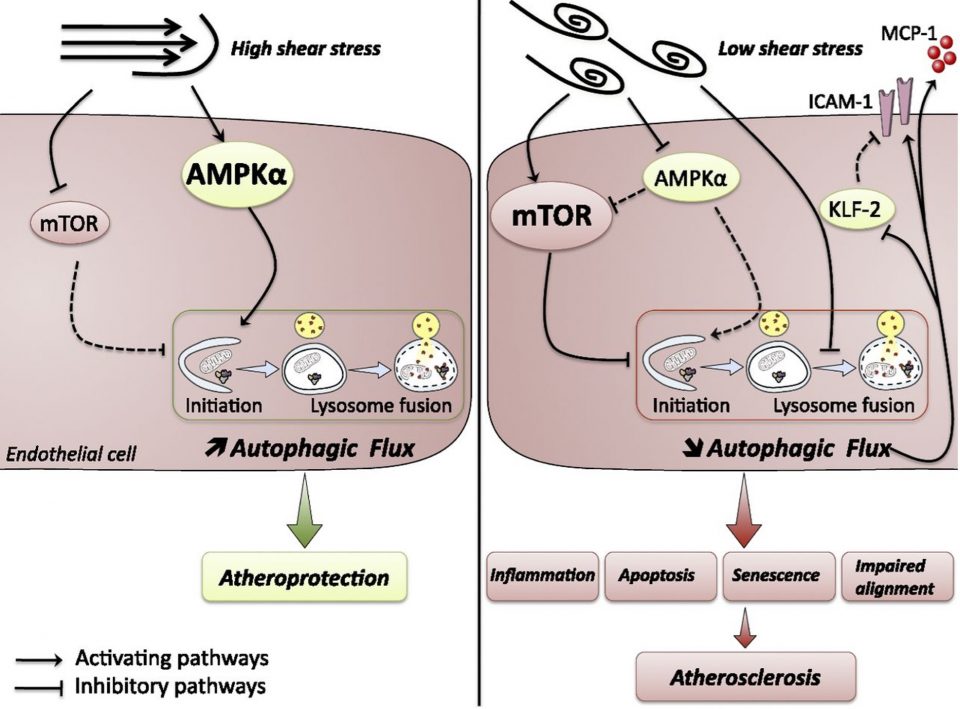
Atherosclerosis is the arterial consequence of metabolic syndrome. It is an inflammatory disease of large arteries which preferentially develops in specific areas of the vasculature, where the blood flow is disturbed and exerts low shear stress, such as arterial bifurcations and curvatures. Conversely, areas exposed to high shear stress are protected from plaque development. We demonstrated that autophagy is activated in high shear stress areas and protects against atherosclerotic plaque formation (Fig. 1; Vion, Kheloufi et al., PNAS 2017). Conversely, autophagic flux is blocked in atheroprone low shear stress areas. This defect is responsible for the preferential plaque formation in atheroprone low shear stress areas, by inducing an endothelial inflammatory, apoptotic, and senescent phenotype. Currently, we are investigating how stimulating endothelial autophagy in atheroprone areas may impact endothelial inflammation and atherosclerotic plaque formation. As the microtubule network is an essential actor in the formation and turnover of autophagosomes, we are testing a strategy of microtubule stabilization, via α-tubulin acetylation, to restore adequate autophagic flux.
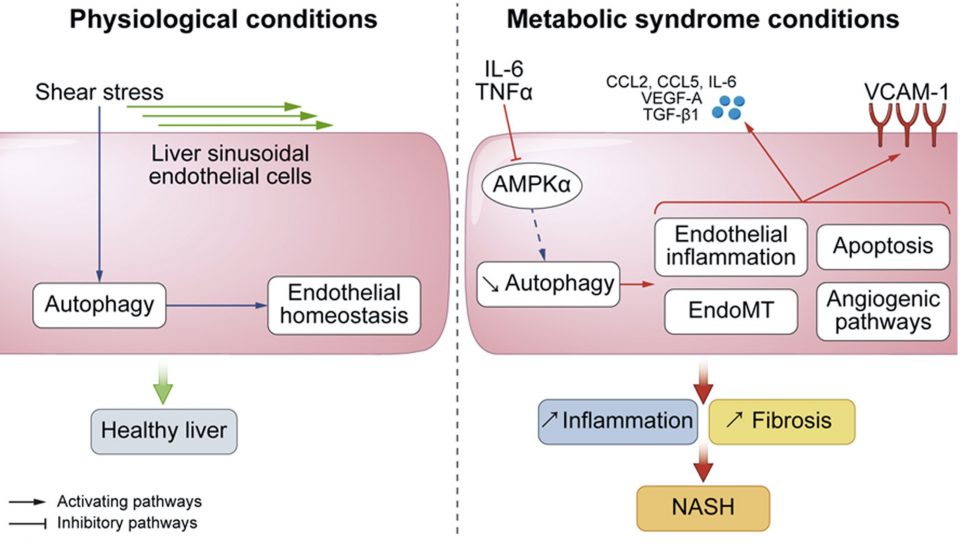
Nonalcoholic fatty liver disease is the liver manifestation of the metabolic syndrome. It encompasses a spectrum of histological lesions ranging from simple steatosis to nonalcoholic steatohepatitis (NASH) which includes, in addition to steatosis, hepatocellular injury, inflammation, and varying degree of fibrosis, and can progress to cirrhosis and liver cancer. We recently demonstrated that autophagy is defective in liver endothelial cells of patients with NASH and that this defect is induced by inflammatory mediators present in the portal blood of patients with metabolic syndrome (Fig. 2). We also demonstrated that deficiency in autophagy induces liver endothelial cell alterations responsible for liver inflammation, cell death and fibrosis, thus promoting NASH development (Hammoutene et al., J. Hepatol 2019). Our work demonstrates that autophagy is a key process involved in endothelial cell homeostasis in a metabolic syndrome setting in different vascular beds. Targeting endothelial autophagy is therefore an attractive strategy for the management of metabolic syndrome related disorders.
Descriptive figure
Publications
Anne-Clémence Vion, Bhama Ramkhelawon, Xavier Loyer, Gilles Chironi, Cecile Devue, Gervaise Loirand, Alain Tedgui, Stéphanie Lehoux, Chantal M Boulanger. Shear stress regulates endothelial microparticle release. Circ Res 2013, 112(10):1323-1333.
Anne-Clemence Vion*, Marouane Kheloufi*, Adel Hammoutene, Johanne Poisson, Juliette Lasselin, Cecile Devue, Isabelle Pic, Nicolas Dupont, Johanna Busse, Konstantin Stark, Julie Lafaurie-Janvore, Abdul I Barakat, Xavier Loyer, Michele Souyri, Benoit Viollet, Pierre Julia, Alain Tedgui, Patrice Codogno, Chantal M Boulanger# and Pierre-Emmanuel Rautou#. Autophagy is required for endothelial cell alignment and atheroprotection under physiological blood flow. Proc Natl Acad Sci USA 2017, 114(41):E8675-E8684. (* and #: equally contributed to the work)
Marouane Kheloufi, Anne-Clemence Vion, Adel Hammoutene, Johanne Poisson, Juliette Lasselin, Cecile Devue, Isabelle Pic, Nicolas Dupont, Johanna Busse, Konstantin Stark, Julie Lafaurie-Janvore, Abdul I. Barakat, Xavier Loyer, Michele Souyri, Benoit Viollet, Pierre Julia, Alain Tedgui, Patrice Codogno, Chantal M. Boulanger and Pierre-Emmanuel Rautou. Endothelial autophagic flux hampers atherosclerotic lesion development. Autophagy 2018, 14(1):173–175.
Adel Hammoutene, Louise Biquard, Juliette Lasselin, Marouane Kheloufi, Marion Tanguy, Anne-Clémence Vion, Jules Mérian, Nathalie Colnot, Xavier Loyer, Alain Tedgui, Patrice Codogno, Sophie Lotersztajn, Valérie Paradis, Chantal M Boulanger and Pierre-Emmanuel Rautou. A defect in endothelial autophagy occurs in patients with non-alcoholic steatohepatitis and promotes inflammation and fibrosis. J Hepatol 2020, 72(3):528-538
Composition de l'équipe
Chantal M. Boulanger, DR InsermXavier Loyer, CR Inserm
Olivier Blanc-Brude, CR CNRS
Dominique Charue, IE Inserm
Cecile Devue, AI Inserm
Fariza Mezine, IE
Michaël Robillard, IE
Pierre-Michaël Coly, Post-doctorant
Shruti Chatterjee, Post-doctorante
Stephane Mazlan, Post-doctorant
Pierre Julia, Clinicien
Sylvain Lejeune, Clinicien
Nicolas Amabile, Clinicien