Intestinal epithelial cell proliferation in physiology and cancer
Inserm U1016-CNRS UMR8104-Université Paris Descartes,Département Développement, Reproduction et Cancer,
Equipe Oncogenèse des épithéliums digestifs,
24 rue du Faubourg Saint Jacques, 75014 Paris - Paris
Site web - beatrice.romagnolo@inserm.fr -
Principal investigator
Béatrice Romagnolo

Research themes
The aim of our group is to identify the molecular mechanisms involved in self-renewal and maintenance of intestinal stem cells (ISC) and to study the associated deregulated mechanisms involved in tumor development.
Historically, our research activity was centered on the study of the Wnt/β-catenin signaling. Our work has helped to demonstrate the fundamental role of this pathway in the proliferation of ISC and progenitors but also in the specification of the Paneth cell lineage which contributes to the ISC niche. Our projects pursue the understanding of the factors required for the functional integrity of ISC by analyzing conditional knockout mutant mice and organoids culture.
A second aim of our research is to study the consequences of deregulation of the Wnt/β-catenin signaling in the gut epithelium. Approximately 80% of sporadic colorectal cancer exhibit abnormal activation of the Wnt/β-catenin signaling due to mutation of the APC gene. We have generated Apc knockout murine models to increase our understanding of cancer development and progression. Our previous work has demonstrated that Apc loss is sufficient to initiate tumorigenesis. Now, we are searching critical Wnt/β-catenin downstream events that act as drivers for intestinal cancer.
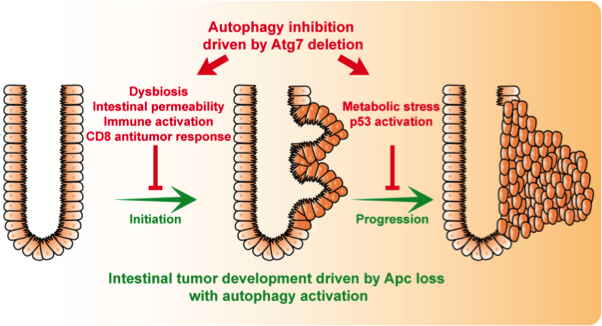
Our recent work highlighted the activation of autophagy in human colon cancers and demonstrated that inhibition of autophagy effectively protect from tumor initiation and progression. Our data demonstrate that the loss of autophagy in the intestinal epithelial cell is responsible for an antitumor immune response and an alteration of the gut microbiota involved in the fight against tumor initiation. In addition, the loss of autophagy in cancer cells suppresses tumor progression via a metabolic stress and cell cycle arrest. Work in our lab continues to explore the significance of these signaling pathways in intestinal tumor biology
Descriptive figure
Publications
– Levy, J., and Romagnolo, B. (2015). Autophagy, microbiota and intestinal oncogenesis. Oncotarget.
– Levy, J., Cacheux, W., Bara, M. A., L’Hermitte, A., Lepage, P., Fraudeau, M., Trentesaux, C., Lemarchand, J., Durand, A., Crain, A. M., et al. (2015). Intestinal inhibition of Atg7 prevents tumour initiation through a microbiome-influenced immune response and suppresses tumour growth. Nat Cell Biol 17, 1062-1073.
– Durand, A., Donahue, B., Peignon, G., Letourneur, F., Cagnard, N., Slomianny, C., Perret, C., Shroyer, N.F., and Romagnolo, B. (2012). Functional intestinal stem cells after Paneth cell ablation induced by the loss of transcription factor Math1 (Atoh1). Proc Natl Acad Sci U S A 109, 8965-8970.
– Peignon, G., Durand, A., Cacheux, W., Ayrault, O., Terris, B., Laurent-Puig, P., Shroyer, N.F., Van Seuningen, I., Honjo, T., Perret, C., Romagnolo, B. (2011). Complex interplay between beta-catenin signalling and Notch effectors in intestinal tumorigenesis. Gut 60, 166-176.
– Andreu, P., Peignon, G., Slomianny, C., Taketo, M.M., Colnot, S., Robine, S., Lamarque, D., Laurent-Puig, P., Perret, C., and Romagnolo, B. (2008). A genetic study of the role of the Wnt/beta-catenin signalling in Paneth cell differentiation. Dev Biol 324, 288-296.
– Andreu, P., Colnot, S., Godard, C., Gad, S., Chafey, P., Niwa-Kawakita, M., Laurent-Puig, P., Kahn, A., Robine, S., Perret, C., and Romagnolo, B. (2005). Crypt-restricted proliferation and commitment to the Paneth cell lineage following Apc loss in the mouse intestine. Development 132, 1443-1451.
Composition de l'équipe
Coralie Trentesaux, doctorante
Marie Fraudeau, AI
Julie Lemarchand, AI