Normal and pathological angiogenesis
- NiceSite web - gilles.pages@uni-cotedazur.fr - 0492031231
Principal investigator
Dr Gilles Pagès

Research themes
Deciphering the mechanisms of resistance to prevent relapses and to develop new therapeutic agents in kidney cancers.
Our project is focused on the mechanisms of resistance to sunitinib, anti-angiogenic therapies used in the first line for metastatic renal clear cell carcinoma (mRCC). We will concentrate on sunitinib.
The intracellular distribution of a drug is a fundamentally important concern for drug efficacy. For the best therapeutic efficacy, the intracellular site of drug localization must correspond to that of the drug target.
Physicochemical properties of drugs like pKa and logP can predictably influence their intracellular localization pattern. In particular, weakly basic drugs defined lipophilic or amphiphilic compounds (logP>1) with a basic moiety (pKa>6) that can become protonated at acidic pH. Protonation prevents their ability to cross membranes. Hence, they are trapped in acidic intracellular compartments such as lysosomes and are qualified as lysosomotropic drugs. Two mechanisms are involved in lysosomes accumulation: 1) passive diffusion; 2) active transport by ABC drug efflux transporters.
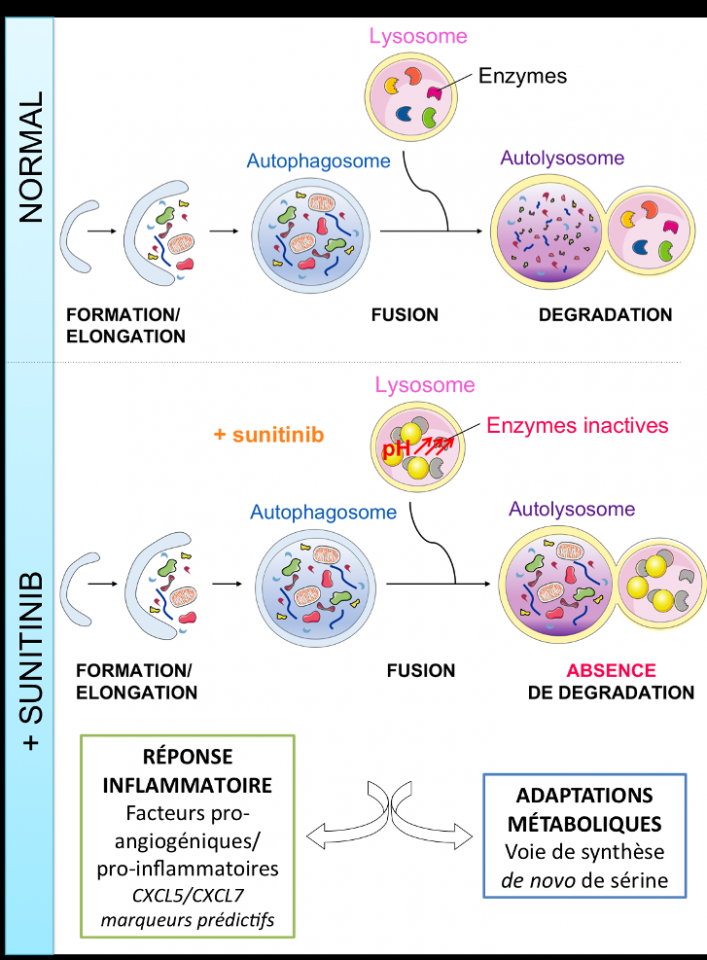
Sunitinib, a new lysomotropic drug leads to autophagy inhibition, inflammatory response and metabolic adaptation.
We demonstrated that i) sunitinib (LogP=5.2, pKa=8.95), initially designed against intra-cytoplasmic targets, is a lysosomotropic drug. It crosses cell membranes via passive diffusion, becomes protonated in lysosomes where it is trapped, ii) the resulting increase of the lysosome pH inhibits lysosomal proteases and consequently leads to an incomplete autophagic process, iii) autophagy inhibition is involved in resistance to sunitinib. Lysosomal drug accumulation can be prevented with agents that alkalinize lysosomes, such as chloroquine, which inhibits lysosomal function by raising their internal pH. (1), (european patent (INSERM transfert, WO2016184793 A1)).
The consequences of lysosomal sequestration and autophagy defect have been poorly addressed. We observed that incomplete autophagic processes stimulate an inflammatory response in transiently and chronically sunitinib-exposed cells via the Nuclear Factor kappa B (NF-kB). We identified two up-regulated pro-inflammatory/pro-angiogenic, CXCL5 and CXCL7 in response to sunitinib. We showed that CXCL5 or CXCL7 plasmatic levels predict sunitinib efficacy of mRCC patients (2;3).
Our transcriptomics, proteomics and metabolomic results reveal that sunitinib-dependent inhibition of autophagy results in de novo serine pathway activation, a mechanism of resistance to sunitinib treatment. We are studying tumor cell metabolic adaptations to compensate the autophagy deficiency and its role in the mechanism of resistance to the drug.
Autophagy and anti-tumoral strategy
This work mainly focused on sutinib, , will be extended to other « multi-target » kinase inhibitors used in second and third line treatment in mRCC (axitinib, pazopanib and cabozantinib) but also to other lysosomotropic drugs and other cancers like breast cancers for which we demonstrated the lysosomal sequestration of lapatinib, an EGFR inhibitor, and the subsequent expression of CXCL5. Thus, deciphering the involvement of autophagy in the mechanisms of resistance to lysosomotropic treatments would allow to understand the therapeutic relevance of activating or inhibiting the autophagic process.
Descriptive figure
Publications
(1) Sandy Giuliano, Yann Cormerais, Maeva Dufies, Renaud Grépin, Pascal Colosetti, Amine Belaid, Julien Parola, Anthony Martin, Sandra Lacas-Gervais, Nathalie Mazure, Rachid Benhida, Patrick Auberger, Baharia Mograbi, Gilles Pagès.
Resistance to sunitinib in renal clear cell carcinoma results from sequestration in lysosomes and inhibition of the autophagic flux.
Autophagy. 2015 Oct 3;11(10):1891-904.
(2) Sandy Giuliano, Maeva Dufies, Papa Diogop Ndiaye, Julien Viotti, Delphine Borchiellini, Julien Parola, Valérie Vial, Yann Cormerais, Mickaël Ohanna, Véronique Imbert, Emmanuel Chamorey, Nathalie Rioux-Leclercq, Ariel Savina, Jean-Marc Ferrero, Baharia Mograbi, Gilles Pagès
Resistance to lysosomotropic drugs used to treat kidney and breast cancers involves autophagy and inflammation and converges in inducing CXCL5.
Theranostics. 2019 Jan 30;9(4):1181-1199.
(3) Maeva Dufies*, Sandy Giuliano*, Julien Viotti, Delphine Borchiellini, Linsay S Cooley, Damien Ambrosetti, Mélanie Guyot, Papa Diogop Ndiaye, Julien Parola, Audrey Claren, Renaud Schiappa, Jocelyn Gal, Antoine Frangeul, Arnaud Jacquel, Ophélie Cassuto, Renaud Grépin, Patrick Auberger, Andréas Bikfalvi, Gérard Milano, Bernard Escudier, Nathalie Rioux-Leclercq, Camillo Porta, Sylvie Negrier, Emmanuel Chamorey, Jean-Marc Ferrero, and Gilles Pagès, (* These authors have contributed equally)
CXCL7 is a predictive marker of sunitinib efficacy in clear cell renal cell carcinomas.
Br J Cancer. 2017 Sep 26;117(7):947-953.
Composition de l'équipe
Dr Gilles Pagès (DR1 INSERM)
Roser Busca (CR1 CNRS)
Manon Campillo (Doctorante)
Anaïs Hagege (Doctorante)
Sandy Giuliano (CRCN)
Philippe Lenormand (CR1 INSERM)
Frédéric Luciano (CR1 INSERM)
Sonia Martial (CR1 CNRS)
Cercina Onesto (MCU)
Julien Parola (Lab Manager / technicien)
Jacques Pouyssegur (Eméritat CNRS)
Olivia Rastoin (Assistante ingénieur)
Manon Teisseire (Master 2)