Apoptosis and Neurogenetics
Laboratoire de Biologie et de Modélisation de la Cellule, CNRS UMR5239ENS de Lyon
46 allée d’Italie 69007 Lyon
Tel: 0033- (0)472728000 - Lyon
Site web - bertrand.mollereau@ens-lyon.fr - 0472728000
Principal investigator
Pr Bertrand Mollereau

Research themes
Equilibrated protein homeostasis or proteostasis requires the precise coordination of folding of newly synthetized proteins, quality control and degradative mechanisms and thereby prevent abnormal aggregation of pathological proteins (Hetz and Mollereau 2014, Mollereau et al. 2016). Neurological disorders such as Alzheimer’s, Parkinson’s, Huntington’s, ALS or prion- like diseases have different clinical outcomes but they all share the accumulation of misfolded pathological proteins and are now classified as protein misfolding diseases (PMD). Protein folding mechanisms that include cytoplasmic and endoplasmic reticulum (ER) chaperones permit the proper folding of newly synthetized proteins. One key mechanism is the unfolded protein response (UPR) that takes place within the ER to ensure folding and proteostasis. In our laboratory we have shown that a preconditioning of the ER using genetics or the drug tunicamycin (ER stress inducer by inhibition of glycosylation) triggered the UPR and conferred neuroprotection in Parkinson’s disease models in Drosophila, mouse and human neuroblastoma cell lines (Mendes et al. 2009; Fouillet et al. 2012). Precisely, we have shown that UPR stimulation induces a neuroprotective pathway that involves autophagy.
We hypothesize that maintaining a physiological ER stress response could be neuroprotective in Parkinson’s disease. In our group, we propose to understand the underlying mechanisms of ER preconditioning and test their contribution in Drosophila, mouse and human cellular models of Parkinson’s disease.
Mechanisms of autophagy inhibition in neurodegeneration
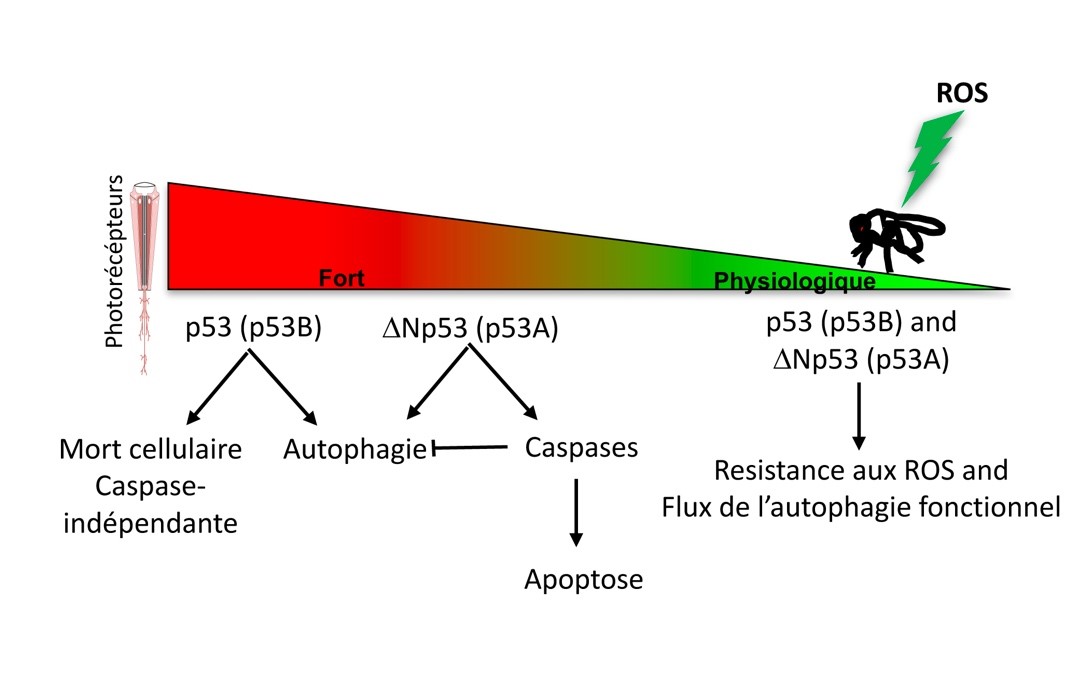
Autophagy is a pro-survival (except in the specific case of ACD) stress response that becomes of paramount importance in long-lived cells in particular in neurons (Boland et al 2018, Napoletano et al in review). The maintenance of autophagy is essential in neuron survival and its inhibition is observed in neurodegeneration. In our laboratory, we are interested in the mechanisms that inhibit autophagy and hypothesize that the apoptosis machinery, which is often activated in neurodegeneration, could contribute to its inhibition. In our recent article in press in Autophagy, we have shown that p53 isoform ΔNp53 (p53A) but not p53 (p53B) induces apoptosis of photoreceptor neurons in Drosophila retina. This apoptosis is associated with high levels of the cleaved effector caspase, Death Caspase-1 (Dcp-1), and concomitant inhibition of the autophagy flux (Robin et al in press and Figure below). Interestingly, a functional autophagy induced by ΔNp53 (p53A) can be restored when initiator or effector caspases are concomitantly inactivated or mutated. Our results suggest that caspases cleave cellular substrates that are required for autophagy execution. The understanding of the mechanisms of mutual inhibition between apoptosis and autophagy will lead to a better understanding of neurodegenerative process to identify potential therapeutic target (Napoletano et al in review, Issa et al 2018).
Descriptive figure
Publications
Boland B, Yu WH, Corti O, Mollereau B, Henriques A, Bezard E, Pastores GM, Rubinsztein DC, Nixon RA, Duchen MR, Mallucci GR, Kroemer G, Levine B, et al. 2018. Promoting the clearance of neurotoxic proteins in neurodegenerative disorders of ageing. Nat Rev Drug Discov 17: 660-88
Fouillet A, Levet C, Virgone A, Robin M, Dourlen P, Rieusset J, Belaidi E, Ovize M, Touret M, Nataf S, Mollereau B. 2012. ER stress inhibits neuronal death by promoting autophagy. Autophagy 8: 915-26
Hetz C, Mollereau B. 2014. Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nat Rev Neurosci 15: 233-49
Issa AR, Sun J, Petitgas C, Mesquita A, Dulac A, Robin M, Mollereau B, Jenny A, Cherif-Zahar B, Birman S. 2018. The lysosomal membrane protein LAMP2A promotes autophagic flux and prevents SNCA-induced Parkinson disease-like symptoms in the Drosophila brain. Autophagy: 1-13
Mendes CS, Levet C, Chatelain G, Dourlen P, Fouillet A, Dichtel-Danjoy ML, Gambis A, Ryoo HD, Steller H, Mollereau B. 2009. ER stress protects from retinal degeneration. Embo J 28: 1296-307
Mollereau B, Rzechorzek NM, Roussel BD, Sedru M, Van den Brink D, Bailly-Maitre B, Palladino F, Medinas DB, Domingos PM, Hunot S, Chandran S, Birman S, Baron T, et al. 2016. Adaptive preconditioning in neurological diseases – Therapeutic insights from proteostatic perturbations. Brain Res
Napoletano F, Baron O, Vandenabeele P, Mollereau B, Fanto M. in review. Regulated cell death and autophagy. Trends in Cell Biology
Napoletano F, Gibert B, Yacobi-Sharon K, Vincent S, Favrot C, Mehlen P, Girard V, Teil M, Chatelain G, Walter L, Arama E, Mollereau B. 2017. p53-dependent programmed necrosis controls germ cell homeostasis during spermatogenesis. PLoS Genet 13: e1007024
Robin M, Issa AR, Santos CC, Napoletano F, Petitgas C, Chatelain G, Ruby M, Walter L, Birman S, Domingos PM, Calvi BR, Mollereau B. in press. Drosophila p53 integrates the antagonism between autophagy and apoptosis in response to stress. Autophagy
Van Den Brink DM, Cubizolle A, Chatelain G, Davoust N, Girard V, Johansen S, Napoletano F, Dourlen P, Guillou L, Angebault-Prouteau C, Bernoud-Hubac N, Guichardant M, Brabet P, et al. 2018. Physiological and pathological roles of FATP-mediated lipid droplets in Drosophila and mice retina. PLoS Genet 14: e1007627
Composition de l'équipe
Nathalie Davoust, Maitre de Conférence ENS (nathalie.davoust-nataf(at)ens-lyon.fr)
Ludivine Walter, Maitre de Conférence Lyon 1 (ludivine.walter(at)ens-lyon.fr)
Florence Jollivet, Ingénieur d’étude CNRS (florence.jollivet(at)ens-lyon.fr)
Marianne Sedru, doctorante (Marianne.sedru(at)ens-lyon.fr)
Haixiu Jin, doctorante (Haixiu.jin(at)ens-lyon.fr)
Victor Girard, doctorant (Victor.girard(at)ens-lyon.fr)