Intracellular transport of viral structures : Adenovirus group
Microbiologie Fondamentale et Pathogenicité,MFP CNRS UMR 5234, Univ. Bordeaux
146 rue Leo Saignat, Bat2B, 1ere etage
33076 Bordeaux cedex, France - Bordeaux
Site web - harald.wodrich@u-bordeaux.fr - 0557571130
Principal investigator
Harald Wodrich (Groupe Adenovirus)

Research themes
The team « intracellular transport of viral structures » at the University of Bordeaux is co-directed by Michael Kann (PR1, Univ. Bordeaux) and Harald Wodrich (INSERM DR2). It hosts two scientifically independent groups, each working on different viral model systems. Our common interest is to understand early (transport/nuclear import) steps in viral infection and the interplay with host restriction factors.
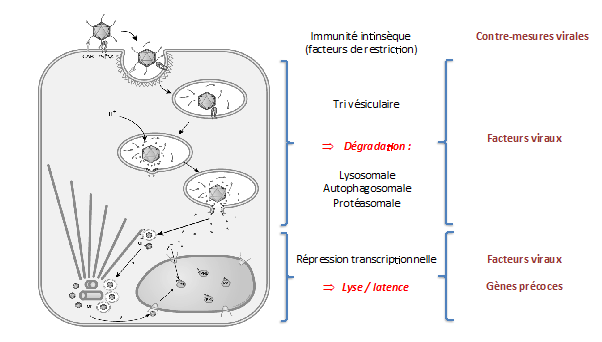
Our group studies adenoviruses (AdV), a non-enveloped DNA virus that replicates in the nucleus of non-dividing cells and is one of the most efficient viruses in terms of genome delivery. AdV enter cells by receptor mediated endocytosis, escape from endosomes followed by transport of the virion to the nuclear pore complex where capsid disassembly and genome release followed by import into the nucleus occur. In our work we develop in vivo imaging approaches to understand the spatio-temporal organization of relevant host-pathogen interactions in living cells. We focus on two essential steps of the entry process. Part of the group aims to understand how AdV docks at the NPC and releases/imports the viral genome into the nucleus (and beyond) and how this is perceived by the cell (Ref. 1-5)
Relevant for the autophagy community, the other part of the group aims to understand how AdV is able to penetrate the endosome to access the cytosol and how the cell responds to this insult. Previous work showed that disassembly cues in the endosome upon AdV endocytosis release the internal capsid protein VI from the entering virion. This protein encodes an N-terminal amphipathic helix responsible for the endosome rupture and subsequent sorting events (Refs. 6-7). Applying live cell imaging we showed that membrane rupture and endosomal escape are two separate events (Refs. 8-9) and that the latter process requires a conserved PPxY motif in protein VI in addition to the amphipathic helix (Ref. 10). More recently we showed that AdV induced membrane damage activates selective autophagy (similar to invasive bacteria) and that the ruptured membrane is targeted by Galectin8 to link the damaged membrane to the autophagic machinery (Ref. 11). Using a virus mutated in the PPxY motif we also showed that the virus uses the motif to recruit ligases of the Nedd4 family (e.g. Nedd4.2) upon membrane damage to prevent autophagic degradation by blocking autophagosome maturation. A defect in autophagosome maturation was also obtained when we depleted Nedd4.2 in non-infected cells, suggesting that the virus prevents Nedd4.2 from engaging in its physiological function in autophagy. Furthermore we showed that AdV’s not only prevent their own autophagic degradation, they also actively exploit the autophagic machinery for efficient retrograde trafficking towards the nucleus in an LC3 assisted manner (Ref. 11).
Following up on this work we are currently interested to understand the molecular basis for i) the role of Nedd4.2 in autophagy regulation and how the virus exploits this function through competitive ligase recruitment via the PPxY motive and ii) how AdV uses the autophagic machinery for nuclear transport acceleration through LC3.
Descriptive figure
Publications
1-Komatsu T, Nagata K, Wodrich H. The Role of Nuclear Antiviral Factors against Invading DNA Viruses: The Immediate Fate of Incoming Viral Genomes. Viruses. 2016 Oct 22;8(10). Review.
2-Komatsu T, Nagata K, Wodrich H. An adenovirus DNA replication factor, but not incoming genome complexes, targets PML nuclear bodies. J Virol. 2015 Nov 25. pii: JVI.02545-15
3-Cassany A, Ragues J, Guan T, Bégu D, Wodrich H, Kann M, Nemerow G and Gerace L. Docking of adenovirus to the nuclear pore complex requires direct interaction with an N-terminal domain of the nucleoporin Nup214. J Virol. 2015 Feb;89(3):1719-30.
4- Schreiner S, Martinez R, Groitl P, Rayne F, Vaillant R, Wimmer P, Bossis G, Sternsdorf T, Marcinowski L, Ruzsics Z, Dobner T, Wodrich H. Transcriptional activation of the adenoviral genome is mediated by capsid protein VI. PLoS Pathog. 2012 Feb;8(2):
5-Wodrich* H*., Cassany A., D’Angelo MA, Guan T., Nemerow G. and Gerace L Adenoviral core protein pVII is translocated into the nucleus by multiple import receptor pathways J. Virol 2006 Oct ; 80(19) :9608-18
6-Wiethoff CM, Wodrich H, Gerace L, Nemerow GR. Adenovirus protein VI mediates membrane disruption following capsid disassembly. J Virol. 2005 Feb;79(4)
7-Martinez R, Schellenberger P, Vasishtan D, Aknin C, Austin S, Dacheux D, Rayne F, Siebert A, Ruzsics Z, Gruenewald K, Wodrich H. The amphipathic helix of adenovirus capsid protein VI contributes to penton release and postentry sorting. J Virol. 2015 Feb;89(4):2121-35.
8-Maier O, Marvin SA, Wodrich H, Campbell EM, Wiethoff CM. Spatiotemporal dynamics of adenovirus membrane rupture and endosomal escape. J Virol. 2012 Oct;86(19):10821-8
9-Martinez R, Burrage AM, Wiethoff CM Wodrich H. High temporal resolution imaging reveals endosomal membrane penetration and escape of adenoviruses in real-time. Methods Mol Biol. 2013;1064:211-26
10-Wodrich H, Henaff D, Jammart B, Segura-Morales C, Seelmeir S, Coux O, Ruzsics Z, Wiethoff CM, Kremer EJ. A capsid-encoded PPxY-motif facilitates adenovirus entry. PLoS Pathog. 2010 Mar 19;6(3)
11-Montespan C, Marvin SA, Austin S, Burrage AM, Roger B, Rayne F, Faure M, Campell EM, Schneider C, Reimer R, Grünewald K, Wiethoff CM, Wodrich H. Multi-layered control of Galectin-8 mediated autophagy during adenovirus cell entry through a conserved PPxY motif in the viral capsid. PLoS Pathog. 2017 Feb 13;13(2)
Composition de l'équipe
Harald Wodrich (GER) (INSERM DR2)
Marie-Edith Lafon (FR) (PU-PH, CHU Bordeaux)
Fabienne Rayne (FR) (MCU Univ. de Bordeaux)
Benoit Roger (FR) (MCU Univ. de Bordeaux)
Irene Carlon (ESP) (Ph. D student, 2014-17)
Noemi Pied (FR) (Ph. D student, 2015-18)
Floriane Lagadec (FR) (Ph. D student, 2016-19)
Aurelien Chuard (FR) (Master 2 student)
Emma Soussens (FR) (Master 1 student)
Membres techniques/administratifs partagés:
Muriel Faure (FR) (Technicienne)
Jessica Ragues (FR) (Technicienne)
Valerie Doussett (FR) (Gestion administrative)