Proteins with tumor suppressor activity
Centre de Recherche en Cancérologie de Marseille (CRCM)Inserm U1068 – CNRS UMR7258 – Institut Paoli-Calmettes – Aix-Marseille Université
Equipe Stress Cellulaire (Dir. Juan Iovanna)
Campus de Luminy Case 915, 163 Avenue de Luminy, 13288 Marseille Cedex 9 - Marseille
Site web - alice.carrier@inserm.fr -
Principal investigator
Alice Carrier

Research themes
Our laboratory is devoted to the study of cell stress response during pancreatic carcinogenesis to increase the understanding of this dramatic cancer and develop novel clinical avenues.
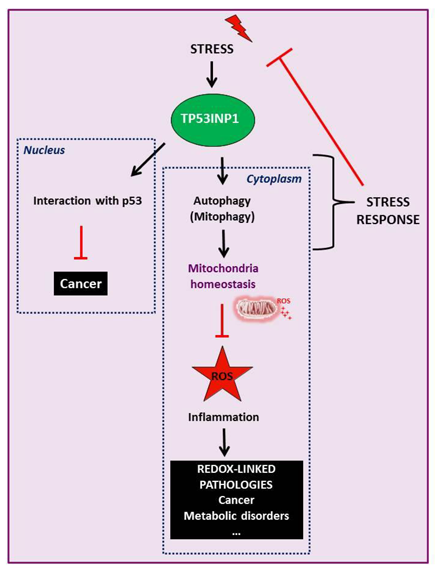
In this context, my group identified a novel stress response protein, the Tumor Protein 53-Induced Nuclear Protein 1 (TP53INP1), which is over-expressed during stress events including inflammation, both in a p53-dependent or -independent manner. We showed that TP53INP1 protein is lost at early stages of cancer progression in several human tissues including pancreas. Mice deficient for TP53INP1 confirmed its tumor suppressive activity associated with its role in oxidative stress control. This anti-tumoral activity occurs both in the nucleus (through the modulation of p53 activity) and in the cytoplasm where TP53INP1 participates in autophagy. Redox control activity of TP53INP1 relies on its ability to participate into mitophagy, a process that eliminates damaged mitochondria which produce high level of reactive oxygen species (ROS).
Interestingly, TP53NP1 paralog (TP53INP2, also known as DOR) also plays a role in autophagy. Both proteins are involved in metabolic syndrome prevention through their impact on cellular metabolism.
Our current projects aim at:
1- Better understand the role of TP53INP1 in mitophagy to assess its function during aging in different organs: bone-marrow (collaboration with Estelle Duprez’s team, specialist of hematopoiesis, CRCM, Marseille) and brain (collaboration with Lydia Kerkerian’s team, specialist of Parkinson’s disease, IBDM Marseille France).
2- Decipher the dysregulations of mitochondrial metabolism in pancreatic cancer, both at the functional (respiration, apoptosis) and the dynamical level (biogenesis, fusion/fission, mitophagy). Our working hypothesis is that mitochondrial metabolism alterations are targetable and could define groups of patients that should benefit from novel therapeutic strategies.
Descriptive figure
Publications
Carrier A. (2016). Metabolic syndrome and oxidative stress: a complex relationship (Forum Editorial). Antioxid Redox Signal, in press.
Amri F, Ghouili I, Amri M, Carrier A*, Masmoudi-Kouki O*. (2016). Neuroglobin protects astroglial cells from hydrogen peroxide-induced oxidative stress and apoptotic cell death. J Neurochem., in press. *co-senior author.
Klionsky et al. (2016). Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12, 1-222.
Saadi H, Seillier M, Carrier A. (2015). The stress protein TP53INP1 plays a tumor suppressive role by regulating metabolic homeostasis. Biochimie, Nov;118:44-50. doi: 10.1016/j.biochi.2015.07.024. Epub 2015 Jul 27. Review.
Seillier M, Pouyet L, N’guessan P, Nollet M, Capo F, Guillaumond F, Peyta L, Dumas JF, Varrault A, Bertrand G, Bonnafous S, Tran A, Meur G, Marchetti P, Ravier MA, Dalle S, Gual P, Muller D, Rutter GA, Servais S, Iovanna JL, Carrier A. (2015). Defects in mitophagy promote redox-driven metabolic syndrome in the absence of TP53INP1. EMBO Molecular Medicine 7, 802-818. DOI – 10.15252/emmm.201404318
Brisson L, Carrier A. (2015). A novel actor in anti-tumoral immunity: the Thymus-Specific Serine Protease TSSP/PRSS16 involved in CD4+ T cell maturation. OncoImmunol, Apr 2;4(9):e1026536. eCollection 2015 Sep.
Brisson L, Pouyet L, N’guessan P, Garcia S, Lopes N, Warcollier G, Iovanna JL, Carrier A. (2015). The Thymus-Specific Serine Protease TSSP/PRSS16 is crucial for the anti-tumoral role of CD4+ T cells. Cell Reports 10, 39-46. DOI – 10.1016/j.celrep.2014.12.009
Shahbazi J, Scarlett CJ, Norris MD, Liu B, Haber M, Tee AE, Carrier A, Biankin AV, Marshall GM, Lock RB, Liu T. (2014). Histone deacetylase 2 and N-Myc reduce p53 protein phosphorylation at serine 46 by repressing gene transcription of Tumor Protein 53 induced nuclear protein 1. Oncotarget 5, 4257-4268.
Saadi H, Seillier M, Sandi MJ, Peuget S, Kellenberger C, Gravis G, Dusetti NJ, Iovanna JL, Rocchi P, Amri M, Carrier A. (2013). Development of an ELISA detecting Tumor Protein 53-Induced Nuclear Protein 1 in serum of prostate cancer patients. Results in Immunology 3, 51-56.
Al Saati T, Clerc P, Hanoun N, Peuget S, Lulka H, Gigoux V, Capilla F, Béluchon B, Couvelard A, Selves J, Buscail L, Carrier A, Dusetti N, Dufresne M. (2013). Oxidative Stress Induced by Inactivation of TP53INP1 Cooperates with KrasG12D to Initiate and Promote Pancreatic Carcinogenesis in the Murine Pancreas. Am J Pathol. 182, 1996-2004.
Seillier M, Peuget S, Gayet O, Gauthier C, N’Guessan P, Monte M, Carrier A, Iovanna JL, Dusetti N. (2012). TP53INP1, a tumor suppressor, interacts with LC3 and ATG8-family proteins through the LC3-interacting region (LIR) and promotes autophagy-dependent cell death. Cell Death Differ 19, 1525-1535.
Composition de l'équipe
Alice Carrier, CR1 CNRS
Rawand Masoud, Post-Doc Fondation ARC
Sophie Lac, Ingénieur FRM
Fatma Amri, Doctorante
Bochra Zidi, Doctorante