Neuroimmunology and peptide therapy
Institut de Science et d’Ingénierie Supramoléculaires (ISIS)8 Allée Gaspard Monge – BP 70028 - Strasbourg
Site web - sylviane.muller@unistra.fr - 0368855109
Principal investigator
Sylviane Muller

Research themes
Our group focuses its research activity on the misdirected immune responses occurring in autoimmune diseases, primarily systemic lupus erythematosus (SLE), and on the discovery of new druggable molecules, mainly peptides and peptidomimetics, designed to specifically immunoregulate these diseases and restore immune tolerance. Some years ago, our group discovered a peptide, which we have called P140, which exhibits remarkable protective effects in MRL/lpr lupus-prone mice that develop a strong lupus-like disease (CNRS patent). In a multicentre, randomized, placebo-controlled phase-IIb study for lupus, P140/LupuzorTM was found to be safe and met its primary efficacy end points, confirming pre-clinical data generated in MRL/lpr mice. It is currently evaluated worldwide in advanced phase-III clinical trials by the Biotech companies ImmuPharma (Mulhouse/London) and Avion Pharmaceuticals LLC (Alpharetta, GA) with SLE patients, the large majority of whom (85%) are young women. P140 has been found to exert an immunomodulatory effect and not immunosuppressive activity, it is stable and not immunogenic, that is a great advantage over the current drug-candidates that are proposed.
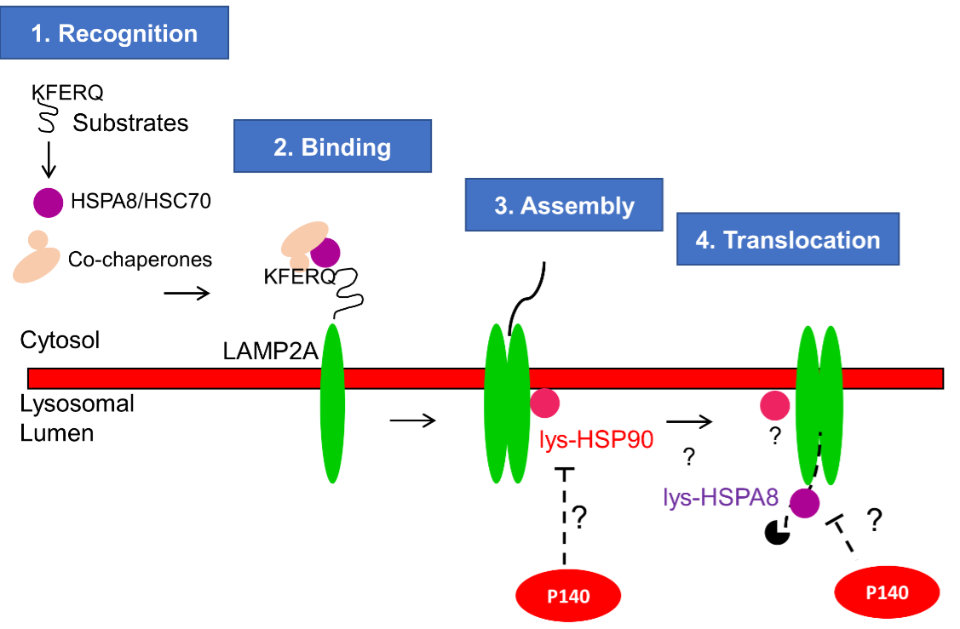
In parallel with these above-mentioned clinical investigations, our research team performed fundamental studies to decipher the mode of action of P140. We found that this therapeutic peptide targets and regulates autophagy processes. Especially, P140 peptide binds the HSPA8/HSC70 chaperone protein and decreases its expression, which we discovered is abnormally enhanced at the plasma membrane of lupus B cells (published in 2009, 2011). The binding of P140 to HSPA8, demonstrated both in vitro and in vivo, destabilizes the interaction of HSPA8 to other chaperone proteins, such as HSP90AA1, which affects HSPA8 biological activity, in particular its protective effects exerted towards major histocompatibility complex (MHC) molecules. This destabilization leads to a significant decrease of the abnormally high MHC molecule expression in antigen-presenting B cells, leading in turn to a beneficial reduction of the hyperactivation of autoreactive T and B cells and in fine, an advantageous reduction of pathogenic autoantibodies secretion. Importantly, we also showed that administration of P140 into lupus-prone mice reduces the autophagic flux in antigen-presenting B cells and corrects the intensity of chaperone-mediated autophagy (CMA) process that is excessive in splenic B cells in lupus. This effect was demonstrated both in vitro and in vivo (published in 2015 and 2020, respectively), thanks to a fruitful collaboration with Ana Maria Cuervo (Bronx, NY). These results then prompted us to explore the effectiveness of P140 in relevant animal models mimicking other inflammatory disorders with an autoimmune origin (Gougerot-Sjögren syndrome, chronic inflammatory demyelinating poly-radiculoneuropathy, inflammatory bowel diseases, as Crohn’s disease and ulcerative colitis) or not autoimmune but of chronic inflammatory origin (asthma, periodontitis). In all these settings, fundamental studies of autophagy processes were performed in our team and often, unexpected findings have emerged (some were patented). Our studies especially revealed the importance of the many defects that alter the quality and activity of lysosomes (hence this major publication in Nature Rev Drug Discov in 2019). In an unanticipated manner, we recently discovered a new class of pathogenic autoantibodies in the serum of lupus patients and mice that react with LAMP2A. We could demonstrate that affinity-purified LAMP2A-reacting autoantibodies accelerate disease progression in genetically predisposed mice (published in 2021). Based on all these findings, we consider today that autophagy represents a pivotal target for therapeutic intervention, especially in conditions of chronic inflammation.
Descriptive figure
Publications
-Page N., Gros F., Schall N., Décossas M., Bagnard D., Briand J.-P. & Muller, S. (2011) HSC70 blockade by the therapeutic peptide P140 affects autophagic processes and endogenous MHCII presentation in murine lupus. Ann. Rheum. Dis. 70, 837-843
-Gros, F., Arnold, J., Page, N., Décossas, M., Korganow, A.-S., Martin, T. & Muller, S. (2012) Macroautophagy is deregulated in murine and human lupus T lymphocytes. Autophagy 8, 1113-1123
-Stricher, F., Macri, C., Ruff, M. & Muller, S. (2013) HSPA8/HSC70 chaperone protein: structure, function and chemical targeting. Autophagy 9, 1937–1954
-Jeltsch-David, H. & Muller, S. (2014) Neuropsychiatric systemic lupus erythematosus: pathogenesis and biomarkers. Nature Rev. Neurol. 10, 579–596
-Gros, F. & Muller, S. (2014) Pharmacological regulators of autophagy and their link with modulators of lupus disease. Brit. J. Pharmacol. 170, 4337-4359
-Macri, C., Wang, F., Tasset, I., Schall, N., Page, N., Briand, J.-P., Cuervo A.M. & Muller, S. (2015) Modulation of deregulated chaperone-mediated autophagy by a phosphopeptide. Autophagy 11, 472-486.
-Wang, F. & Muller, S. (2015) Manipulating autophagic processes in autoimmune diseases: a special focus on modulating chaperone-mediated autophagy, an emerging therapeutic target. Front. Immunol. 6, 252
-Bianco, A. & Muller, S (2015) Nanomaterials, autophagy and lupus disease. ChemMedChem.11, 166-174
-Muller, S. & Radic, M. (2016) Oxidation and mitochondrial origin of NET DNA in lupus. Nature Med. 22, 126-127
-Muller, S., Brun, S., René, F., De Sèze, J., Loeffler, J.-P. & Jeltsch-David, H. (2017) Autophagy in neuroinflammatory diseases. Autoimmunity Rev. 16, 856-874
-Brun, S., Schall, N., Bonam, S.R, Bigaut, K., Mensah-Nyagan, A.G., de Sèze, J. & Muller, S. (2018) An autophagy-targeting peptide to treat chronic inflammatory demyelinating polyneuropathies. J. Autoimmunity 92, 114-125
-Li, B., Wang, F., Schall, N. & Muller, S. (2018) Rescue of autophagy and lysosome defects in salivary glands of MRL/lpr mice by a therapeutic phosphopeptide. J. Autoimmunity 90, 132-145
-Bonam, S.R, Wang, F. & Muller, S. (2018) Autophagy: a new concept in autoimmunity regulation and a novel therapeutic option. J. Autoimmunity 94, 16-32
-Retnakumar, S.V. & Muller, S. (2019) Pharmacological autophagy regulators as therapeutic agents for inflammatory bowel diseases. Trends Mol. Med. 25, 516-537
-Bonam, S.R, Wang, F. & Muller, S. (2019) Lysosomes as a therapeutic target. Nature Rev. Drug Discov. 18, 923-948
-Bonam, S.R., Tschan M.P., Bayry, J. & Muller, S. (2020) Progress and challenges in the use of MAP1LC3 as a legitimate marker for measuring dynamic autophagy in vivo. Cells 9, 1321
-Bonam, S.R., Muller, S. Bayry, J. & Klionsky, D.J. (2020) Autophagy as an emerging target for COVID-19: lessons from an old friend, chloroquine. Autophagy 16, 2260-2266
-Wang, F., Tasset, I., Cuervo, A.M. & Muller, S. (2020) In vivo remodeling of altered autophagy-lysosomal pathway by a phosphopeptide in lupus. Cells 9, 2328
-Voynova, E., Lefebvre, F., Qadri, A. & Muller, S. (2020) Correction of autophagy impairment inhibits pathology in the NOD.H-2h4 mouse model of primary Sjögren’s syndrome. J. Autoimmunity 108, 102418
-Wilhelm, M., Bonam, S.R., Schall, N., Bendorius, M., Korganow, A.-A., Lumbroso C. & Muller, S. (2021) Implication of a lysosomal antigen in the pathogenesis of lupus erythematosus. J. Autoimmunity 120, 102633
-Daubeuf, F., Schall, N., Petit-Demoulière, N., Frossard, N. & Muller, S. (2021) An autophagy modulator peptide prevents lung function decrease and corrects established inflammation in murine models of airway allergy. Cells 10, 2468
Composition de l'équipe
Sylviane Muller, Directeur de recherche émérite au CNRS, Professeur à l’Institut d’Etudes Avancées de l’Université de Strasbourg (USIAS), titulaire de la Chaire d’Immunologie thérapeutique, Directeur de l’Institut du Médicament de Strasbourg (IMS)
Thomaz Vieira, Professeur University of Minas Gerais (Brésil) année sabbatique
Laura Talamini, Post-doc (Italie)
Sruthi V. Retnakumar, Doctorante (Inde)
Nicolas Schall, Assistant-ingénieur (CDD)
Cindy Verdot, Assistant-ingénieur (CDD)
Antoine Gioux, Assistant-ingénieur (CDD)
Dylan Mastrippolito, Etudiant M2 Sciences du médicament et des produits de santé, (spécialité Pharmacologie & toxicologie)
* Hélène Jeltsch-David Chargé de recherche CNRS, détachée à l’INSERM depuis 2020