Lipness (Lipoproteins and lipid transfers in sterile and septic inflammation).
UFR des Sciences de Santé7 Bd Jeanne d’arc - Dijon
Site web - david.masson@u-bourgogne.fr - 0380393352
Principal investigator
David MASSON, PU-PH

Research themes
Mitophagy and inflammatory response (Main investigators : C.THOMAS, P.E. CHARLES, M. BLOT)
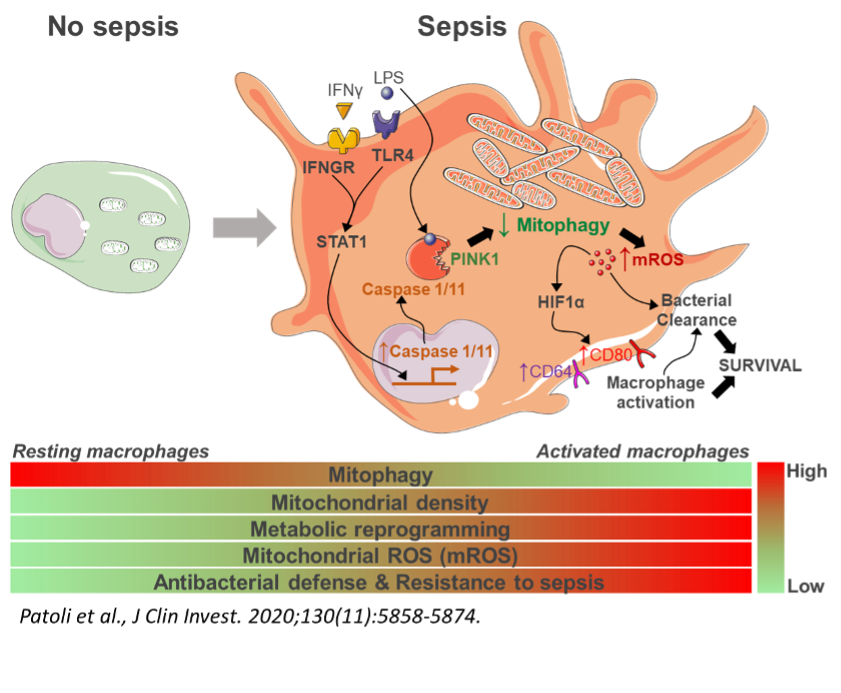
Mitophagy is a mitochondria-dedicated autophagy allowing clearance of damaged mitochondria in the cell. Our investigations aim to unravel the signaling pathway resulting to the inhibition of mitophagy by LPS and IFNγ in macrophages. We investigate whether this inhibition of mitophagy occurs in macrophages and polynuclear neutrophils in the context of bacterial infection such as sepsis or experimental models of pneumonia and whether modulation of mitophagy may affect the inflammatory and bactericidal responses.8,9
Our recent works demonstrated that the activation of the STAT1 signaling pathway leads to the inhibition of mitophagy in myeloid cells by inducing a inflammatory caspases 1 & 11-dependent degradation of PINK1. This inhibition of mitophagy appeared to be a protective factor against sepsis triggered by gram-negative bacteria. We were able to bring a proof of concept of the therapeutic potential of this discovery using pharmacological modulators of mitophagy. We also highlighted its interest as a biomarker of sepsis (Patoli et al., JCI, 2020). We are currently pursuing several projects in the continuity of this work both in the field of septic inflammation (sepsis and septic shock) and sterile inflammation (atherosclerosis and aging). Septic inflammation: 1) MIMIC clinical trial aims at studying mitophagy in myeloid cells in ICU patients with or without sepsis. 2) We are currently developing a project which aims at vectorising pharmacological modulators of mitophagy in order to specifically modulate the activation of macrophages as well as their inflammatory response. Sterile inflammation: The ANR project STATmiNADage (collaboration with Prof. J. Auwerx, EPFL, Lausanne) aims to study the interaction between the STAT1 signaling pathway, mitophagy and NAD metabolism in myeloid cells in atherosclerosis related to aging.
Other Main Research Axis of the Lipness Team :
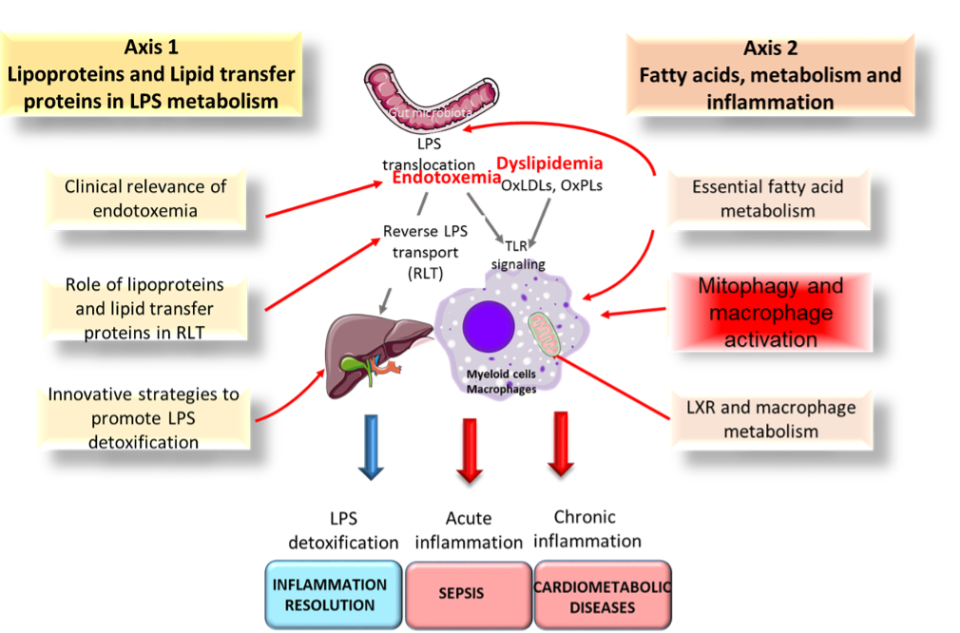
Deciphering the role of lipoproteins in the Reverse Lipopolysaccharide Transport pathway (Main investigators: L.LAGROST, V.DECKERT, T.GAUTIER, M. NGUYEN)
Lipopolysaccharides (LPS) bind and activate Toll like receptor 4 at the surface of immune cells, leading to the release of pro-inflammatory cytokines and to inflammation. Alternatively, bacterial blebs forming large LPS aggregates can be disrupted and molecular transfer of LPS towards lipoproteins can occur. It results in its neutralization and elimination back to the liver, namely the reverse LPS transport pathway (RLT). Recent observations suggest that PLTP and CETP, as members of the lipid transfer/lipopolysaccharide binding protein gene family play a driving role in RLT, thus modulating inflammation and innate immunity. 1–3
Role of bile acids in the neutralization/detoxification of LPS (Main investigators: (S. MANDARD, T. GAUTIER)
Some evidence indicates that LPS molecules are removed from the body through biliary excretion as the final step of the reverse LPS transport pathway. While some previous works have documented the biophysical interaction of endotoxins with purified bile acids, its pathophysiological consequence in terms of proinflammatory capacity of LPS is not completely clear. We aim at better understanding the link between bile acid metabolism and neutralization/detoxification of LPS.
Endotoxemia in human populations and pathophysiological consequences in sepsis and septic shock (Main investigators (J.P. QUENOT, B. BOUHEMAD, L.LAGROST)
The incidence of severe sepsis and septic shock is about 15% of all admission in ICU and the hospital mortality is 25% and 49%, respectively. Despite significant advances in our understanding of the pathophysiology of severe sepsis and septic shock, attempts to translate these finding into novel treatments and improved outcomes for patients have been disappointing. One main objective of the Lipness team is to determine to which extent LPS neutralization and elimination may constitute a new and relevant approach to improve clinical outcome of septic shock.4
Role of GLP-1 and insulin in the LPS-mediated inflammatory response (Main investigator : J.GROBER)
In healthy subjects, moderate endotoxemia has been associated with increased insulin sensitivity on the short term whereas insulin resistance can occur at later time points. We have recently shown that LPS is able to increase circulating levels of glucagon-like peptide-1 (GLP-1), an incretin involved in insulin secretion stimulated by glucose (GSIS).5
Modulation of Fatty acid metabolism in macrophages and inflammation (Main investigators : D.MASSON, C. THOMAS)
Phospholipids are continuously remodeled through deacylation and reacylation by the opposite actions of phospholipase A2, and lysophospholipid acyl-transferases (LPLATs). LPLATs affect both the PUFA content of phospholipids and the availability of free fatty acids such as arachidonic acid used for eicosanoid synthesis. By using specific mouse models and samples from human patients, we assess the impact of phospholipid and fatty acid metabolism in myeloid cells on inflammation and atherosclerosis development. 6,7
Polyunsaturated fatty acids and prevention of metabolic syndrome, associated endotoxemia and inflammation (Main investigators : J. BELLENGER, S. BELLENGER, M. NARCE).
Metabolic syndrome (MS) is tightly associated with low grade inflammation, endotoxemia and intestinal microbiota modulation. A decrease of the n-6/n-3 ratio by n-3 PUFA-enriched diets can exert beneficial effects during obesity and insulin-resistance in rodent models and microbiome changes could improve intestinal integrity and reduce hepatic and systemic inflammation. We recently showed (by microbiome transplantation) that endogenously synthesized n-3 PUFAs exert beneficial effects during MS. We are now focused on 1) evaluating the effects of fatty acids on intestinal barrier 2) deciphering molecular mechanisms involved in the relative contribution of n-3-enriched microbiota in the prevention of MS. A special attention is paid on the mucus layer in terms of strengthening the intestinal barrier integrity by n-3 fatty acids.10
Descriptive figure
Publications
1. Deckert V, Lemaire S, Ripoll P-J, de Barros J-PP, Labbé J, Borgne CC-L, Turquois V, Maquart G, Larose D, Desroche N, Ménétrier F, Le Guern N, Lebrun LJ, Desrumaux C, Gautier T, Grober J, Thomas C, Masson D, Houdebine L-M, Lagrost L. Recombinant human plasma phospholipid transfer protein (PLTP) to prevent bacterial growth and to treat sepsis. Sci Rep. 2017;7:3053.
2. Sali W, Patoli D, Pais de Barros J-P, Labbé J, Deckert V, Duhéron V, Le Guern N, Blache D, Chaumont D, Lesniewska E, Gasquet B, Paul C, Moreau M, Denat F, Masson D, Lagrost L, Gautier T. Polysaccharide Chain Length of Lipopolysaccharides From Salmonella Minnesota Is a Determinant of Aggregate Stability, Plasma Residence Time and Proinflammatory Propensity in vivo. Front Microbiol. 2019;10:1774.
3. Nguyen M, Pallot G, Jalil A, Tavernier A, Dusuel A, Le Guern N, Lagrost L, Pais de Barros J-P, Choubley H, Bergas V, Guinot P-G, Masson D, Bouhemad B, Gautier T. Intra-Abdominal Lipopolysaccharide Clearance and Inactivation in Peritonitis: Key Roles for Lipoproteins and the Phospholipid Transfer Protein. Front Immunol. 2021;12:622935.
4. Dargent A, Pais De Barros J-P, Ksiazek E, Fournel I, Dusuel A, Rerole AL, Choubley H, Masson D, Lagrost L, Quenot J-P. Improved quantification of plasma lipopolysaccharide (LPS) burden in sepsis using 3-hydroxy myristate (3HM): a cohort study. Intensive Care Med. 2019;45:1678–1680.
5. Lebrun LJ, Lenaerts K, Kiers D, Pais de Barros J-P, Le Guern N, Plesnik J, Thomas C, Bourgeois T, Dejong CHC, Kox M, Hundscheid IHR, Khan NA, Mandard S, Deckert V, Pickkers P, Drucker DJ, Lagrost L, Grober J. Enteroendocrine L Cells Sense LPS after Gut Barrier Injury to Enhance GLP-1 Secretion. Cell Rep. 2017;21:1160–1168.
6. Ménégaut L, Jalil A, Thomas C, Masson D. Macrophage fatty acid metabolism and atherosclerosis: The rise of PUFAs. Atherosclerosis. 2019;291:52–61.
7. Ménégaut L, Thomas C, Jalil A, Julla JB, Magnani C, Ceroi A, Basmaciyan L, Dumont A, Goff WL, Mathew MJ, Rébé C, Dérangère V, Laubriet A, Crespy V, Barros J-PP de, Steinmetz E, Venteclef N, Saas P, Lagrost L, Masson D. Interplay between Liver X Receptor and Hypoxia Inducible Factor 1α Potentiates Interleukin-1β Production in Human Macrophages. Cell Reports [Internet]. 2020 [cited 2020 May 19];31. Available from: https://www.cell.com/cell-reports/abstract/S2211-1247(20)30618-5
8. Patoli D, Mignotte F, Deckert V, Dusuel A, Dumont A, Rieu A, Jalil A, Van Dongen K, Bourgeois T, Gautier T, Magnani C, Le Guern N, Mandard S, Bastin J, Djouadi F, Schaeffer C, Guillaumot N, Narce M, Nguyen M, Guy J, Dargent A, Quenot J-P, Rialland M, Masson D, Auwerx J, Lagrost L, Thomas C. Inhibition of mitophagy drives macrophage activation and antibacterial defense during sepsis. J Clin Invest. 2020;
9. Blot M, Jacquier M, Pauchard L-A, Rebaud C, Marlin C, Hamelle C, Bataille A, Croisier D, Thomas C, Jalil A, Mirfendereski H, Piroth L, Chavanet P, Bensoussan D, Laroye C, Reppel L, Charles P-E. Adverse Mechanical Ventilation and Pneumococcal Pneumonia Induce Immune and Mitochondrial Dysfunctions Mitigated by Mesenchymal Stem Cells in Rabbits. Anesthesiology. 2022;136:293–313.
10. Bidu C, Escoula Q, Bellenger S, Spor A, Galan M, Geissler A, Bouchot A, Dardevet D, Morio B, Cani PD, Lagrost L, Narce M, Bellenger J. The Transplantation of ω3 PUFA-Altered Gut Microbiota of fat-1 Mice to Wild-Type Littermates Prevents Obesity and Associated Metabolic Disorders. Diabetes. 2018;67:1512–1523.
Composition de l'équipe
CHERCHEURS
MASSON David (PU-PH) HDR*
THOMAS Charles (MCU) – responsable projets mitophagie, Charles.Thomas@u-bourgogne.fr
BELLENGER Jérome (MCU)
BLOT Mathieu (MCUPH)*
BOUHEMAD Bélaid (PUPH) HDR*
BOURGEOIS Thibaut (ater)
CHARLES Pierre-Emmanuel (PU-PH) HDR*
DECKERT Valérie (CR Inserm)*
GAUTIER Thomas (CRCN- INSERM ) HDR*
GROBER Jacques (MCU) HDR
LAGROST Laurent (DR Inserm)
MANDARD Stéphane (MCU)*
NARCE Michel (PU) HDR
QUENOT Jean-Pierre (PUPH) HDR*
GUINOT Pierre-Grégoire (PUPH) HDR
PERSONNELS ITA
BELLENGER Sandrine (IR – uB)
BERGAS Victoria (TR-uB) 50%
BLANCHE Jean-Marc (Adj uB)
JARDEL Honorine (AI INSERM)
LE GUERN Naig (TR – INSERM)*
PAIS DE BARROS Jean-Paul (IR – INSERM) 50%*
PILOT Thomas (IE INSERM)*
PROUKHNITZKY Lil (AI – UBFC)*
ROBLET Loic (adj – uB)
ETUDIANTS ET POST-DOCTORANTS
BENKHALED Anissa (M2R)
BOURRAGAT Amina (PhD 3ème année Inserm)
EL HAMWI Amar (PhD – 1ère année UB)
LE NEINDRE Aymeric (PhD – 3 ème année)
LELEU Damien (post-doc CHU)
LOISEAU Mélanie (PhD – 1ère année CHU)
MEGENAUT Louise (post-doc CHU)
MEUNIER-BEILLARD Nicolas (PhD 2ème année CHU)
NGYEN Maxime (post-doc CHU)*
PALLOT Gaetan (PhD – 3ème année)
VAN DONGEN Kevin (PhD – 3ème année)*
VOUILLOZ Adrien (PhD – 2ème année UBFC)
MANGIN Léa (M2R)*